Although the formation of a thrombus or clot within a blood vessel is important for maintaining hemostasis, pathological thrombosis can occur and cause deep vein thrombosis (DVT), pulmonary embolism (PE), stroke, or myocardial infarction (MI). The images below show an overview of the process for diagnosing DVT and PE, respectively.
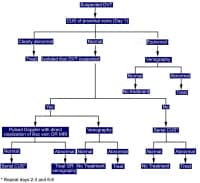 Diagnosis of deep vein thrombosis during pregnancy.
Diagnosis of deep vein thrombosis during pregnancy. 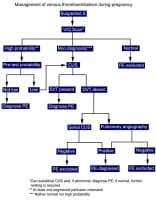 Diagnosis of pulmonary embolism during pregnancy.
Diagnosis of pulmonary embolism during pregnancy. Antithrombotic agents (ie, anticoagulants and thrombolytic agents) are first-line therapy for pathological thromboses. Anticoagulants interrupt the coagulation cascade to prevent thrombus formation and extension while endogenous thrombus lysis occurs. They are available in oral or parenteral forms. Thrombolytic agents promote thrombus lysis and are administered parenterally.
Warfarin is an oral anticoagulant that interferes with liver synthesis of vitamin K-dependent clotting factors, which leads to depletion of factors II (prothrombin), VII, IX, and X and prolongation of clotting times (ie, international normalized ratio [INR]). Rivaroxaban is an orally active factor Xa inhibitor that prolongs prothrombin time (PT) and activated partial thromboplastin time (aPTT). Dabigatran is an orally administered direct thrombin inhibitor that results in prolongation of the aPTT. Unlike warfarin, the pharmacokinetics of rivaroxaban and dabigatran are predictable; thus, routine monitoring of coagulation parameters is not required when one of these agents is used. Although warfarin has been used extensively in pregnancy, the safety and efficacy of the newer oral agents (rivaroxaban or dabigatran) in pregnancy has not been established.
The most commonly used parenteral anticoagulants inactivate thrombin and/or factor Xa without depleting circulating levels of clotting factors. Unfractionated heparin, low molecular weight heparin (LMWH), heparinoids, synthetic pentasaccharide inhibitors (eg, fondaparinux), and direct thrombin inhibitors (ie, hirudin and argatroban) belong to this category.
Thrombolytic agents mediate the dissolution of fibrin clots by promoting the conversion of plasminogen to plasmin, which causes degradation of fibrin to fibrin degradation products. Traditional thrombolytic agents include streptokinase (SK), anisoylated plasminogen streptokinase activator complex (APSAC), urokinase, and recombinant tissue plasminogen activator (t-PA).
Evidenced indications for the use of antithrombotic agentsIndications of antithrombotic use have been recently published and include the following:[1]
Acute and chronic venous thromboembolism (including pulmonary embolism )Atrial fibrillationValvular and structural heart diseaseIschemic strokeAcute coronary syndromesPeripheral artery occlusive diseasePregnancy is associated with 4 times increased risk of venous thromboembolism (VTE) and the risk increases to 14-fold during puerperium.[2] This risk further increases if an underlying thrombophilia is present. PE remains a leading cause of maternal mortality in the Western world.[3] The risk in pregnant women is 5 times higher than in nonpregnant women of the same age.[4]
Anticoagulant therapy is indicated in pregnancy for the treatment acute VTE, valvular heart disease, and for the prevention of pregnancy-related complications in women with antithrombin deficiency or antiphospholipid antibody syndrome (APLAs) and other thrombophilias who have had prior VTE.[5, 6]
NextPathophysiologyNormal pregnancy is associated with a hypercoagulable state due, at least in part, to increased serum levels of procoagulants, such as factor II,[7] VII,[7, 8] VIII,[7] X,[7, 9, 10] XII,[9, 10] and fibrinogen.[7, 11] In addition, protein S levels decrease during pregnancy,[12] and increased resistance to activated protein C is observed in the second and third trimesters of pregnancy.
Concomitantly, serum plasminogen activator inhibitor-1 (PAI-1) and placental plasminogen activator inhibitor-2 (PAI-2) increase with pregnancy which leads to a decreased fibrinolytic state.{Ref12}[13] Venous stasis resulting from pressure of the gravid uterus on the inferior vena cava and decreased venous tone are additional predisposing factors to VTE.
PreviousNextEpidemiology of Venous Thromboembolism in PregnancyThe incidence of pregnancy-associated VTE is estimated at 1 in 500 to 2000 deliveries (0.05-0.2%).[14, 15] The risk is 4-fold to 50-fold higher in pregnant women than in nonpregnant women and is highest during puerperium.[16, 17] The incidence of VTE in puerperium and pregnancy is 7.19 and 0.97 per 1000 women, respectively.[18] The risk of pregnancy-related VTE is particularly high in heterozygous carriers of factor V Leiden (4-fold to 16-fold increase),[19] the prothrombin mutation (15-fold increase),[20] and women with antiphospholipid antibodies (5% incidence).{{Ref 6}
Most (approximately 85%) of DVT of the lower extremity occur on the left side during pregnancy. This is attributed to the more tortuous course of the venous drainage of the left leg through the pelvis and compression of the left common iliac vein by the overlying right iliac artery.[21]
PreviousNextPrevention and Treatment of Venous ThromboembolismThe following recommendations are part of the Eighth American College of Chest Physicians (ACCP) Conference on Antithrombotics and Thrombolytics Therapy: Evidence Based Guidelines.[22]
Table 1. ACCP Risk Factors and Recommendations (Open Table in a new window)
Risk FactorRecommendationsWomen with a single episode of VTE associated with a transient risk factor that is no longer presentClinical surveillance and anticoagulant prophylaxis postpartum*Women with a single episode of VTE and thrombophilia (confirmed laboratory abnormality) and a strong family history of thrombosis who are not receiving long-term anticoagulants Prophylactic or intermediate-dose LMWH or unfractionated heparin (UFH), plus postpartum anticoagulation for at least 6 wk (for a total minimum duration of therapy of 6 mo) Women with antithrombin deficiency and no previous VTEAntepartum and postpartum prophylaxisWomen with thrombophilia (other than antithrombin deficiency) and no previous VTEClinical surveillance or prophylactic LMWH or UFH and anticoagulant prophylaxis postpartum*Women with multiple (≥ 2) episodes of VTE who are not receiving long-term anticoagulantsProphylactic, intermediate-dose or adjusted-dose UFH or adjusted-dose LMWH followed by long-term anticoagulation postpartumWomen with multiple (≥ 2) episodes of VTE who are receiving long-term anticoagulantsAdjusted-dose UFH or LMWH followed by resumption of long-term anticoagulation postpartumAll women with previous DVT, antenatal and postpartumUse of graduated elastic compression stockingsWomen with recurrent pregnancy loss (≥ 3 miscarriages) and women with severe or recurrent preeclampsia, placental abruption, or otherwise unexplained intrauterine growth retardation Screen for thrombophilia and antiphospholipid antibodiesWomen with antiphospholipid antibody syndrome and a history of multiple (≥ 2) early pregnancy losses or ≥ 1 late pregnancy losses, preeclampsia, intrauterine growth retardation (IUGR), or abruptionAntepartum aspirin plus prophylactic or intermediate-dose UFH or LMWHWomen with APLAs and a history of VTE who are usually receiving long-term oral anticoagulation therapyAdjusted-dose LMWH or UFH therapy plus low-dose aspirin and resumption of long-term oral anticoagulation therapy postpartum* If the previous risk factor is pregnancy or estrogen-related or additional risk factors (such as obesity) are present, antenatal anticoagulant prophylaxis is recommended.The following are ACCP recommendations for antithrombotic agents.[22] In pregnant women with acute VTE, the following 2 alternative approaches are reasonable:
Subcutaneous LMWH can be used initially and for long-term treatment with dose adjustment based on monitoring of anti-Xa levels.Intravenous (IV) UFH bolus is followed by continuous infusion to maintain aPTT in the therapeutic range for at least 5 days, followed by subcutaneous LMWH or dose-adjusted subcutaneous UFH for the remainder of pregnancy.LMWH is preferred over UFH for the prevention and treatment of VTE because of ease of use and better efficacy and safety profile. Anticoagulants should be administered for at least 6 weeks postpartum (for a minimum total duration of therapy of 6 mo).
In women receiving dose-adjusted LMWH or UFH therapy, discontinuing anticoagulant therapy 24 hours prior to elective induction of labor is recommended. If spontaneous labor occurs, careful monitoring of aPTT or anti-Xa levels is required. If aPTT is markedly prolonged near delivery, protamine sulfate may be required to reduce the risk of bleeding.
PreviousNextAnticoagulation During Pregnancy in Patients With Valvular Heart DiseaseWomen with valvular heart disease who are pregnant or planning to conceive require careful evaluation and management. Physiologic changes associated with pregnancy are poorly tolerated in some cases of valvular heart disease. These include aortic stenosis, mitral regurgitation, aortic regurgitation with New York Heart Association (NYHA) class 3-4 symptoms, mitral stenosis with NYHA class 2-4 symptoms, valvular heart disease that results in severe pulmonary hypertension, and left ventricular (LV) dysfunction with an ejection fraction (EF) less than 40%. Patients with mechanical prosthetic valve requiring anticoagulation are exposed to special risks during pregnancy.[23] Therefore, whenever possible, symptomatic or severe valvular lesions should be addressed before conception.
TreatmentAnticoagulation is recommended in most pregnant patients with a mechanical prosthetic heart valve, whereas those with a bioprosthetic valve do not require anticoagulation. Warfarin (Coumadin) is more efficacious than UFH for thromboembolic prophylaxis of pregnant women with mechanical valves.[24] Unfortunately, warfarin therapy in the first trimester of pregnancy is associated with a substantial increase in fetal anomalies, and anticoagulation with any agent is associated with an increased incidence of fetal wastage (approximately 30%), prematurity (approximately 45%), and low birth weight (approximately 50%).[25, 26, 22]
In a systematic review of fetal and maternal outcome of pregnancy with mechanical heart valves, the regimen associated with the lowest risk of valve thrombosis (3.9%) was warfarin throughout pregnancy. However, its use throughout pregnancy was associated with warfarin embryopathy in 6.4% of live births. The substitution of heparin at or prior to 6 weeks, and continued until 12 weeks, eliminated the risk of warfarin embryopathy. Using heparin only from 6-12 weeks' gestation was associated with an increased risk of valve thrombosis (9.2%).
In 2002, the US Food and Drug Administration (FDA) issued a warning that LMWH was not recommended for thromboprophylaxis in pregnant women with prosthetic heart valves. However, a consensus panel concluded that this recommendation was based on studies in which underdosing or inadequate monitoring of LMWH occurred.[27] Consequently, the panel supports the use of LMWH as a treatment option with monitoring of anti-Xa levels. Seshadhri et al reviewed 120 articles and concluded that LMWH, compared with UFH, may be a safe and effective agent in patients with mechanical prosthetic heart valves.[28] However, large-scale, randomized trials are warranted.
PreviousNextRecommendationsRecommendations for anticoagulation of pregnant women with prosthetic heart valves is based on the Eighth ACCP Conference on Antithrombotics and Thrombolytics and are as follows:[22]
Adjusted dose twice daily (bid) subcutaneous LMWH throughout pregnancy to achieve a peak anti-Xa level of 1-1.2 U/mL 4 hours after injection or Adjusted dose bid subcutaneous UFH throughout pregnancy to achieve mid-interval aPTT at least twice control or an anti-Xa level of 0.35-0.70 U/mL or UFH or LMWH (as above) until 13 weeks' gestation, change to warfarin until the middle of the third trimester, and then restart UFH or LMWHLong-term anticoagulants should be resumed postpartum with all regimens.
High-risk women with prosthetic heart valves, such as women with LV dysfunction and women with prior thromboembolic episodes, should have the addition of low-dose aspirin 75-162 mg/d.
PreviousNextMaternal and Fetal Complications Secondary to AnticoagulationUse of anticoagulants in the breastfeeding motherHeparin and LMWHs are not secreted into breast milk and can be safely administered to women who are breastfeeding.[29]
Two reports show that maternal administration of warfarin does not induce an anticoagulant effect in the breastfed infant. Thus, women using this drug should be encouraged to breastfeed.[29, 30]
Fetal complications of anticoagulants during pregnancyWarfarin crosses the placenta and can cause both fetal bleeding and teratogenicity, with the latter occurring mainly during the first trimester.[31]
Neither UFH[32] nor LMWH[33, 34] cross the placenta; therefore, these agents do not cause fetal bleeding or teratogenicity, although bleeding at the uteroplacental junction and fetal wastage is possible.
Maternal complications of anticoagulants during pregnancyThe rate of major bleeding in patients treated with UFH therapy is 2%.[35]
Approximately 3% of patients receiving UFH develop immune thrombocytopenia (so called, heparin-induced thrombocytopenia [HIT]), which predisposes to venous and arterial thrombosis.[36]
Heparin-induced osteoporosis causes vertebral fracture in 2-3% of patients and significant reduction in bone density is seen in about 30% of patients receiving long-term UFH. LMWH causes less osteoporosis and HIT than UFH.[37, 38, 39, 40, 41]
Previous, Anticoagulants and Thrombolytics in Pregnancy





0 comments:
Post a Comment