![]()
Overview
The presence of cardiovascular disease in pregnant women poses a difficult clinical scenario in which the responsibility of the treating physician extends to the unborn fetus. Profound changes occur in the maternal circulation that have the potential to adversely affect maternal and fetal health, especially in the presence of underlying heart conditions. Up to 4% of pregnancies may have cardiovascular complications despite no known prior disease.
NextPhysiological Changes During Pregnancy and Puerperium
Pregnancy has a profound effect on the circulatory system. Most of these hemodynamic changes start in the first trimester, peak during the second trimester, and plateau during the third trimester. Cardiac output increases 30-50% secondary to increase in blood volume and heart rate.[1, 2] Blood pressure decreases by 10-15 mm Hg owing to a decrease in systemic vascular resistance caused by the creation of a low resistance circuit by the placenta and vasodilatation.[3] Additionally, heart rate normally increases by 10-15 beats per minute. The hematocrit level decreases due to a disproportionate increase in plasma volume that exceeds the rise in red cell mass.[1, 2]
During the third trimester, cardiac output is further influenced by body position, where the supine position causes caval compression by the gravid uterus. This leads to a decrease in venous return, which can cause supine hypotension of pregnancy. Stroke volume normally increases in the first and second trimester and decreases in the third trimester. This decrease is due to partial vena cava obstruction.
The delivery and immediate postpartum period is associated with further profound and rapid changes in the circulatory system. During delivery, cardiac output, heart rate, blood pressure, and systemic vascular resistance increase with each uterine contraction.[1, 4, 5] Delivery-related pain and anxiety aggravate the increase in heart rate and blood pressure.
Immediately postpartum, the delivery of the placenta increases afterload by removing the low resistance circulation and increases the preload by returning placental blood to the maternal circulation. This increase in preload is accentuated by the elimination of the mechanical compression of the inferior vena cava. Blood loss is typically 300-400 mL during vaginal delivery and 500-800 mL during cesarean delivery. These changes can place an intolerable strain on an abnormal heart, necessitating invasive hemodynamic monitoring and aggressive medical management.[6] Postpartum, the cardiac output is typically reduced for 2-6 weeks.[7, 8]
PreviousNextCardiovascular Evaluation During Pregnancy
The patient's history is an essential part of the initial risk assessment and should include information on the baseline functional status and previous cardiac events because these are strong predictors of peripartum cardiac events. The strongest predictors include the following:
Any prior cardiac eventCyanosis or poor functional classLeft-sided heart obstructionVentricular dysfunction
Left-sided heart obstruction includes valve disease or hypertrophic cardiomyopathy (aortic valve area 2, mitral valve area 2, or left ventricular outflow tract peak gradient >30 mm Hg). Impaired ventricular function is significant when the ejection fraction is below 40%.[9] Prior events of interest also include treatment for heart failure, TIA or stroke, or arrhythmia.
The 2011 update to the American Heart Association guideline for the prevention of cardiovascular disease (CVD) in women recommends that risk assessment at any stage of life include a detailed history of pregnancy complications. Gestational diabetes, preeclampsia, preterm birth, and birth of an infant small for gestational age are ranked as major risk factors for CVD.[10]
Many of the normal symptoms of pregnancy, such as dyspnea on exertion, orthopnea, ankle edema, and palpitations, are also symptoms of cardiac decompensation. However, angina, resting dyspnea, paroxysmal nocturnal dyspnea, or a sustained arrhythmia are not expected with pregnancy and warrant a further diagnostic workup.[6] Almost all pregnant women develop physiologic murmurs, which are usually soft, midsystolic murmurs heard along the left sternal border usually caused by functional pulmonary stenosis due to increased transvalvular flow.
Physical signs commonly seen with pregnancy are jugular venous distension, an apical S3, basal crackles, prominent left and right ventricular apical impulses, exaggerated heart sounds, and peripheral edema. Diastolic murmurs are rare with pregnancy despite the increased blood flow through the atrioventricular valves;[11] their presence should prompt further diagnostic evaluation.[12] Systolic murmurs more than 2/6 in intensity, continuous murmurs, and murmurs that are associated with symptoms or electrocardiographic changes should also prompt further investigation such as echocardiography.[12]
Electrocardiography offers low-cost screening that may identify the need for further study if findings otherwise appear benign. In pregnancy, the axis can shift right or left but usually stays in the normal range.[13] During normal pregnancy, multiple changes can be seen such as increased R wave amplitude in leads V1 and V2, T wave inversion in lead V2, and a small Q wave and inverted P wave in lead III.[11] Pregnancy is associated with a higher rate of maternal arrhythmias,[14] ranging from 73-93% in some studies.[15] {Ref16}
If impaired functional status is a concern or the patient's history is unreliable, baseline oxygen saturation and low-level exercise testing (targeted to 70% of age-predicted maximum heart rate; 70% of 220 – age) with oxygen monitoring and oxygen consumption may be helpful. Cardiac catheterization should be avoided in pregnancy and should be reserved only for situations in which therapeutic intervention is being considered.[16] Findings such as ventricular hypertrophy, evidence of a prior myocardial infarction or ischemia, atrial enlargements, conduction abnormalities, or arrhythmias should prompt a more extensive workup.[17]
PreviousNextPregnancy and Valvular Heart Disease
Valvular heart disease in pregnancy is relatively infrequent, with an incidence of less than 1%.[9] In the developed world, valvular disease in women of childbearing age is often congenitally acquired.[18] Rheumatic heart disease, myxomatous degeneration, previous endocarditis, and bicuspid aortic valves are also encountered. Pregnancy complicated by valvular heart disease tends to have a favorable prognosis if risks are appropriately managed. Management of the pregnant woman with a heart condition requires special expertise, and patients with high-risk conditions should be referred to centers specialized in their care.
The American College of Cardiology and the American Heart Association (AHA/ACC) classifies maternal and fetal risk during pregnancy based on the type of valvular abnormality and the New York Heart Association (NYHA) functional classification.[17] This is depicted below. Decreased functional status (NYHA class II or higher) and specific valvular conditions including mitral stenosis and aortic stenosis are associated with increased neonatal complications such as premature birth, intrauterine growth restriction, respiratory distress syndrome, intraventricular hemorrhage, and death.
If medical intervention is necessary during pregnancy, the lowest adequate therapeutic dose of the required medication should be used.[19] Medications such as hydralazine, methyldopa, digoxin, adenosine, and procainamide can be safely used in pregnancy.[7] Angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers, amiodarone, and nitroprusside are contraindicated during pregnancy regardless oftheindication.[7, 6] Most other medications carry a potential risk to the fetus and should only be used when the maternal benefit outweighs the fetal risk.[20]
In a woman with valvular disease, a short, pain-free labor and delivery helps to minimize hemodynamic changes. Hemodynamic monitoring, including continuous monitoring of oxygen saturation, ECG, and arterial pressure should be under surveillance. Rarely, pulmonary artery wedge pressures, and cardiac output, may be indicated in severe disease. Fetal monitoring is another means of assessing the adequacy of cardiac treatment because fetal distress is an indicator of impaired cardiac output.
Women with valvular disease should undergo a vaginal delivery with adequate pain control as cesarean delivery results in greater hemodynamic changes and blood loss and should be reserved for obstetric indications. In certain patients, especially those with mitral or aortic stenosis, delivery should be aided by forceps or vacuum-assisted techniques to avoid the sudden rise in systemic vascular resistance and drop in systemic venous return that occurs with maternal pushing.
Endocarditis prophylaxis remains a controversial issue in vaginal and cesarian deliveries. The ACC/AHA guidelines recommend against prophylaxis in cesarian deliveries. In vaginal deliveries, the ACC/AHA does not recommend prophylaxis, but discretion is left to the physician who is caring for high-risk patients.
Early studies reported a low incidence of bacteremia with vaginal delivery.[21, 22] However, more recent studies found, in some circumstances, the incidence can be as high as 5-19%.[23, 24] When endocarditis occurs during pregnancy, maternal and fetal mortality rates are 22% and 25%, respectively.[25]
In patients with underlying valvular heart disease, many centers administer prophylaxis antibiotics using the AHA guidelines of ampicillin 2.0 g IM or IV plus gentamicin 1.5 mg/kg (not to exceed 120 mg) given at initiation of labor or within 30 min of a cesarean delivery, followed by ampicillin 1 g IM or IV or amoxicillin 1 g orally 6 hours later. For patients allergic to penicillin, vancomycin 1.0 g IV over 1-2 hours is recommended instead.
Risk classification in pregnancy and heart disease
The following conditions are considered high maternal and fetal risk:
Severe aortic stenosis with or without symptomsAortic regurgitation with NYHA class III or IV symptomsMitral stenosis with NYHA class II, III, or IV symptomsMitral regurgitation with NYHA class III or IV symptomsAortic valve disease, mitral valve disease, or both resulting in pulmonary hypertension with a pulmonary pressure greater than 75% of systemic pressures Aortic valve disease, mitral valve disease, or both with left ventricular ejection fraction less than 40%Maternal cyanosisAny valve disease with NYHA class III or IV symptoms
The following conditions are considered low maternal and fetal risk:
Asymptomatic aortic stenosis with a mean transvalvular gradient of less than 50 mm Hg and normal left ventricular systolic function Aortic regurgitation with NYHA class I or II symptoms and normal left ventricular systolic functionMitral regurgitation with NYHA class I or II symptoms and normal left ventricular systolic functionMitral valve prolapse with no regurgitation or with mild-to-moderate regurgitation and normal left ventricular systolic functionMild-to-moderate mitral stenosis (mitral valve area >1.5 cm2, gradient Mild-to-moderate pulmonary valve stenosisPreviousNextSpecific Valvular LesionsMitral stenosis
Mitral stenosis (MS) is almost always due to rheumatic heart disease; other possibilities include congenital mitral stenosis, systemic lupus erythematosus, rheumatoid arthritis, atrial myxoma, malignant carcinoid, and bacterial endocarditis.[26, 27]
The pregnancy-induced increase in plasma volume leads to elevated left atrial and pulmonary vein pressures. This may cause pulmonary edema and lead to symptoms of dyspnea, orthopnea, and paroxysmal nocturnal dyspnea. The increased heart rate observed during pregnancy, decreases diastolic filling time, which further increases left atrial pressure. This may provoke atrial arrhythmias, which shortens diastolic filling time even further.[26, 27]
Despite the high risk of complications, maternal mortality is generally less than 1%[28] and appears to be confined to patients with severe mitral stenosis and NYHA class IV symptoms.[25, 29] Fetal complications include preterm delivery and intrauterine growth restriction.[26] Fetal mortality increases with worsening maternal functional capacity and may be as high as 30% when the mother has NYHA class IV symptoms.[30]
Management of the pregnant woman with mitral stenosis should include reducing the heart rate and left atrial pressure by restricting physical activity and administering a beta-adrenergic receptor blocker.[31] In patients with atrial fibrillation, digoxin may also be useful as well as safe for control of ventricular rate.[19] If a calcium channel blocker is needed, verapamil is preferred over diltiazem.[32] Left atrial pressure can also be reduced by decreasing blood volume through salt restriction and the use of oral diuretics. Aggressive use of diuretics should be avoided to prevent hypovolemia and reduction of uteroplacental perfusion.[33]
In patients with severe symptoms, percutaneous balloon mitral valvuloplasty performed during the second trimester has been associated with normal subsequent deliveries and excellent fetal outcomes.[34] This procedure is preferable to open mitral valve commissurotomy, which carries a fetal loss rate of 10-30%.[35] Commissurotomy is reserved for patients with severe mitral stenosis who are refractory to optimal medical therapy and are not suitable candidates for percutaneous balloon mitral valvuloplasty.[7] Ultrasound examinations to monitor fetal growth are recommended monthly. Fetal monitoring with nonstress tests and amniotic fluid levels should be considered in women with poor functional status or during any acute changes in maternal symptoms.
Ultrasonography to monitor fetal growth is recommended monthly. Fetal non-stress testing should be considered in women with poor functional status or during any acute changes in maternal symptoms.
Management in labor usually focuses on avoiding rapid changes in hemodynamic status and avoidance of tachycardia. Vaginal delivery is usually well tolerated. Epidural anesthesia is useful in avoiding the catecholamine-induced tachycardia.[36] Epidural anesthesia is usually preferred over spinal anesthesia because it has a slower onset of blockade and therefore more controlled hemodynamic changes.[36] Cesarean delivery should be performed for obstetrical indications only, as the hemodynamic changes from cesarean delivery may be more detrimental postpartum than those that occur during vaginal delivery.
Chronic mitral regurgitation
Chronic mitral regurgitation in pregnancy is usually due to mitral valve prolapse or rheumatic heart disease.[26, 37, 29] It is usually well tolerated during pregnancy due to the decrease in systemic vascular resistance.[6] Asymptomatic patients do not require specific therapy during pregnancy. In the presence of symptomatic left ventricular dysfunction with hemodynamic abnormalities, diuretics, digoxin, hydralazine, and nitrates can be administered. Surgery for mitral valve repair or replacement during pregnancy has been associated with a high incidence of fetal loss[38] and should be considered only in patients with severe symptoms not controlled by medical therapy.
Aortic stenosis
Aortic valve stenosis in young women is usually related to rheumatic fever or congenital valvular abnormalities, especially bicuspid aortic valves.[39] Most patients with mild aortic stenosis have a favorable outcome.[26, 40] Moderate-to-severe stenosis has an increased risk of cardiac and obstetrical complications.
Cardiac complications in patients with aortic stenosis include heart failure (7%), arrhythmias (2.5%), and ischemic events (2.5%).[41] The increased cardiac output related to pregnancy can lead to heart failure, and the increased heart rate in the third trimester can lead to ischemic events. The potential obstetrical complications include preeclampsia or other hypertensive related disorders, premature birth, and small-for-gestational-age births.[42] These patients should be followed closely in the third trimester, including ultrasound to monitor fetal growth.
Patients with congenital aortic valve stenosis have increased risk of congenital heart disease in the fetus of 4%[41] ; these patients may benefit from fetal echocardiography at 20-22 weeks.
Anesthesia management at delivery is controversial because patients may not tolerate the decrease in preload and afterload that occurs with regional anesthesia. Labor is not contraindicated. An assisted second stage is appropriate to minimize significant changes in the cardiac output that can occur during prolonged labor. Ideally, women with symptomatic aortic stenosis should have surgical intervention prior to pregnancy. Some experts offer pregnancy termination in the face of symptomatic severe aortic stenosis. The patient should be counseled about the maternal risks of pregnancy.
Chronic aortic regurgitation
Aortic regurgitation in young women may be due to a bicuspid aortic valve, dilated aortic annulus (as in Marfan syndrome), or previous endocarditis. The reduced systemic vascular resistance of pregnancy reduces the volume of regurgitant blood. Isolated aortic regurgitation can usually be managed with vasodilators such as hydralazine or nifedipine and diuretics[17] along with salt restriction. Women with an abnormal functional capacity or left ventricular dysfunction have an increased risk of abnormal maternal outcomes.[7] If possible, surgery, if indicated, should be delayed until after delivery to avoid the high risk of fetal loss.[38]
Symptomatic patients and patients with left ventricular dysfunction may benefit from hemodynamic monitoring during labor and delivery. Extensive clinical and echocardiographic assessment should be performed before conception in women with aortic regurgitation due to Marfan syndrome because this syndrome predisposes women to aortic dissection during pregnancy.[43, 44]
Prosthetic heart valves
Risks of prosthetic valves in pregnancy include thromboembolism, structural valve failure, bleeding secondary to anticoagulation, and infection. Women with mechanical prosthetic heart valves require lifelong anticoagulation to prevent valve thrombosis. Women with bioprosthetic valves do not require anticoagulation; however, some theoretical concern exists that the increased blood volume associated with pregnancy may hasten valve deterioration in these patients. Overall, the durability of bioprosthetic valves is approximately 10 years and has not be shown to be less in women who have had pregnancies.[45]
Valve thrombosis is a life-threatening condition, and, therefore, women with mechanical prosthetic valves require anticoagulation. Warfarin crosses the placenta, and its use is associated with embryopathy, fetal loss, and fetal cerebral hemorrhage. The ACC/AHA guidelines suggest using unfractionated heparin or low molecular weight heparin (LMWH) from 6-12 weeks of gestation and then conversion back to warfarin until 36 weeks of gestation, at which time women are converted back to heparin or LMWH.[46] The 2008 American College of Chest Physicians guidelines support maintaining LMWH as a treatment option in these women.[47]
Treatment options including risks and benefits to both mother and fetus should be discussed with the patient, and patient preference is an important part of the decision. However, much debate has occurred regarding the use of low molecular weight heparin (LMWH) in pregnancy due to a suspected increased risk of valve thrombosis.[48] Recent studies have shown that rates of thromboembolic events are low if LMWH dosing is adjusted according to anti-factor Xa levels.[49] If LMWH is to be used in pregnancy, women are recommended to receive 1mg/kg twice daily, and anti-factor Xa levels are followed with a goal of 1.0-1.2 U/ml 4-6 hours after injection.[47]
Preconceptually, patients should be counseled to continue warfarin until pregnancy is confirmed. Early diagnosis of pregnancy is important to decrease the fetal risks of warfarin. Patients should be converted to unfractionated heparin or LMWH in the first trimester as soon as pregnancy is determined.
Timing of delivery should be anticipated so that anticoagulation can be managed to decrease the risk of bleeding. If urgent delivery is necessary while the mother is receiving warfarin, reversal of anticoagulation is appropriate to avoid massive hemorrhage; however, the benefit must be weighed against risk of thrombosis.
PreviousNextPregnancy and Congenital Heart Disease
With advances in management of congenital heart disease, almost 85% of patients with congenital heart disease now survive to adulthood and childbearing age.[50, 51] Traditionally, these patients were advised against pregnancy; however, as our understanding of the unique issues facing this population has improved, many of those limitations have been removed. Although some patients with congenital heart disease may not tolerate the hemodynamic changes of pregnancy, many women have sufficient cardiac reserve to safely carry a pregnancy to term.[52]
Death is a rare occurrence during pregnancy in women with congenital heart disease;[3, 53, 54] however, maternal and fetal complications are substantial.[9, 55] The risk of the infant having a congenital abnormality ranges from an average of 3% to about 50% in autosomal dominant single gene defects such as Marfan syndrome.[52] Simple lesions with minimal hemodynamic changes, such as small atrial septal defects, carry a low risk of maternal deterioration or fetal complications. On the other hand, Eisenmenger syndrome carries a significant risk of deterioration to the mother including death. Cyanotic congenital heart diseases carry the highest risk to the fetus, from intrauterine growth restriction to spontaneous abortion.[52]
Management should start early with preconception risk stratification using appropriate clinical and laboratory investigations. Emphasis should be placed on the risk factors that are highlighted below. The patient should be informed about multiple issues, including the expected rate of complications and risk of congenital anomalies in her offspring. Fetal ultrasonographic screening should be offered, with level II ultrasound at 17-18 weeks and fetal echocardiography at 18-22 weeks.
Patients at low risk should receive routine obstetric care and endocarditis prophylaxis as indicated.[52] Patients at moderate risk usually tolerate pregnancy well; however, they do pose certain management difficulties. Significant anomalies should be assessed for possible repair prior to pregnancy,[56] and medical management should be modified to avoid certain harmful effects to the fetus. High-risk patients should be counseled against pregnancy, and, in the event of pregnancy, offering early termination should be considered. Both moderate-risk and high-risk patients should be followed at tertiary centers with maternal fetal specialists who have extensive experience in dealing with pregnancy in congenital heart disease.
Women with moderate-risk or high-risk lesions, especially cyanotic lesions, have an increased risk of fetal growth restriction and should be followed with monthly ultrasounds for fetal growth. In cases of maternal decompensation, fetal monitoring should also be done to ensure fetal well being. Women with moderate-risk or high-risk lesions, especially cyanotic lesions, have an increased risk of fetal growth restriction and should be followed with monthly ultrasound examinations for fetal growth. If growth restriction occurs, then these pregnancies need to be followed with twice-weekly NSTs and weekly evaluation of amniotic fluid volumes. In cases of maternal decompensation, fetal monitoring should also be performed to ensure fetal well being. Close collaboration between both cardiac and obstetric teams is needed for optimal care.[52]
Decisions about timing and mode of delivery must be made well in advance of labor. Vaginal delivery is preferred because it causes smaller shifts in blood volume, less hemorrhage, fewer clots, and fewer infections.[57] Cesarean delivery is indicated only for obstetric reasons.[11]
Oxygen should be administered to all hypoxemic patients with arterial saturation monitoring for patients with cyanotic conditions, pulmonary hypertension, and cardiac dysfunction. Hemodynamic monitoring with arterial lines or Swan-Ganz catheters can be performed if necessary. Epidural anesthesia with adequate volume preloading is the preferred method for labor anesthesia, except in defects when a decrease in systemic vascular resistance is hazardous.
Endocarditis prophylaxis is controversial. The AHA guidelines suggest that prophylaxis is unnecessary except in cases of prosthetic heart valves or surgically constructed systemic to pulmonary shunts.[58] Due to the devastating effects of endocarditis, some clinicians recommend prophylaxis in vaginal deliveries except in patients with isolated secundum type of atrial septal defect or those who are more than 6 months from surgical repair of septal defects or surgical ligation of patent ductus arteriosus.[11]
Pregnancy and congenital heart disease
High maternal risk conditions are as follows:
Poor functional class before pregnancy (NYHA functional classification II or more) or cyanosisImpaired systemic ventricular function (ejection fraction Mitral valve stenosis (area 2), aortic valve stenosis (area 2), left ventricular outflow tract peak pressure gradient greater than 30 mm Hg before pregnancyPreconception history of adverse cardiac events such as symptomatic arrhythmia, stroke, transient ischemic attack, and pulmonary edema Marfan syndromeEisenmenger syndromePulmonary hypertension
Moderate maternal risk conditions are as follows:
Repaired tetralogy of Fallot without significant pulmonic stenosis or regurgitation[59] Complex congenital heart disease with the anatomic right ventricle serving as systemic ventricleMild mitral or aortic valve stenosisCyanotic lesions without pulmonary hypertensionFontan type circulationUncorrected coarctation of the aorta
Low maternal risk conditions are as follows:
Small ventricular septal defectsAtrial septal defectsBicuspid aortic valve without stenosis, regurgitation, or aortic dilationRepaired coarctation of the aortaPreviousNextSpecific Congenital Defects
Atrial septal defects are usually well tolerated during pregnancy. In a study by Actis et al, miscarriages, preterm delivery, and cardiac deterioration occurred more frequently in patients who did not undergo surgical correction of their defect prior to pregnancy.[60] Generally, decisions concerning pregnancy in this group should be made on an individual basis considering functional status, pulmonary hypertension, and the presence of additional cardiac lesions.[11]
Isolated small ventricular septal defects (VSD) are well tolerated; however, larger defects are associated with an increased risk of congestive heart failure, arrhythmias, and pulmonary hypertension.[61, 62] Closure of the VSD prior to the onset of pulmonary hypertension or ventricular dysfunction reduces the incidence of complications to that of the general population.[11] The incidence of VSD in the offspring ranges from 4-11%.[63] In patients with pulmonary hypertension, shunt reversal and cyanosis can occur secondary to reduced blood pressure during pregnancy and delivery. These patients may require vasopressors and close monitoring throughout their pregnancy.
Outcomes of patent ductus arteriosus (PDA) in pregnancy with left-to-right shunting is usually favorable; however, clinical deterioration and congestive heart failure have been reported.[61, 62] The rate of occurrence of PDA in the offspring is less than 1%.[64] In patients with pulmonary hypertension, reversal of the shunt with cyanosis can occur due to decreased blood pressure and may be prevented by the use of vasopressors.[11]
Coarctation of the aorta is usually well tolerated in pregnancy.[65, 66] Severe hypertension, heart failure, and aortic dissection have been reported.[61, 65, 67] Complications are less likely in cases of repaired coarctation; however, hypertension is still common, especially with the presence of increased coarctation gradient.[68] Aortic dissection has been reported in pregnant patients with repaired coarctation.[69] The incidence of congenital heart disease in the offspring is reported to be 3-4%.[65, 66] Beta-blockers are the treatment of choice for hypertension in this group of patients due to the added effect of protection against aortic dissection.
In uncorrected tetralogy of Fallot (TOF), the fall in systemic vascular resistance associated with pregnancy may lead exacerbate the right-to-left shunt. Poor prognostic factors include maternal hematocrit above 60%, arterial oxygen saturation below 80%, elevated right ventricular systolic pressure and syncopal episodes.[11] Cyanosis is associated with an increased rate of spontaneous abortion, preterm delivery, and intrauterine growth restriction.[61] Full surgical correction reduces the risk of complications to that of the general population and, therefore, is recommended prior to conception.[61]
Palliative procedures with residual pulmonic regurgitation, right ventricular dilation and dysfunction, and right ventricular outflow obstruction are risk factors for arrhythmia and heart failure during pregnancy.[70] Close hemodynamic and arterial saturation monitoring during delivery are recommended for cyanotic or symptomatic patients.[11] Cardiac defects have been reported to occur in 3-17% of offspring.[63]
Women who were born with complex congenital cardiac lesions such as transposition of the great vessels, tricuspid atresia, and single ventricle are now reaching reproductive age due to the success of Mustard, Senning, or Fontan procedures. Multiple studies have reported successful pregnancies in these patients.[71, 72, 73, 74] Complications during pregnancy include maternal arrhythmia, heart failure, and myocardial infarction.[75, 74] A higher rate of preterm delivery and growth restriction exists.[75, 74] Women who have undergone repair for transposition of the great vessels are generally advised that pregnancy is safe, but a multidisciplinary approach is needed. Women who have undergone a Fontan procedure have in the past been advised to avoid pregnancy. With increasing reports of successful pregnancies, this recommendation is being challenged by some.[76]
Eisenmenger syndrome is usually associated with increased maternal morbidity and mortality reaching 40%, usually occurring between the first days and a few weeks after deliver.[61, 77, 78] Fetal loss, preterm delivery, intrauterine growth restriction, and perinatal death are also more frequent.[61, 79] Patients in this group should be advised against pregnancy and, in the event of accidental pregnancy, early abortion can be offered.
In patients who chose to continue with their pregnancy, close management by experts is essential. Early hospitalization to restrict activity and ensure close monitoring may be necessary. Spontaneous vaginal delivery with continuous hemodynamic monitoring is preferred. Due to the possibility of prolonged induction and the need for an emergency cesarean delivery, a planned cesarean delivery may be considered.[11]
PreviousNextPregnancy and CardiomyopathyHypertrophic cardiomyopathy
Hypertrophic cardiomyopathy (HCM) has been considered a relatively rare disease in pregnant women. However, the diagnosis is increasing in frequency due to increased awareness and improved screening.
HCM may be identified by a systolic ejection heart murmur that increases with Valsalva maneuver, by increased QRS voltage on the ECG, and/or by abnormal wall thickness and Doppler blood flow by echocardiography.
Clinical presentation of this disease is widely variable and pregnancy may increase the morbidity and mortality associated with this condition.[80, 81] Syncope may occur from left ventricular outflow tract obstruction, arrhythmias, or myocardial ischemia or infarction. Baseline functional status of the patient is an important determinant of the clinical outcome of these women during pregnancy. Clinical deterioration during pregnancy is uncommon, occurring in less than 5% of previously asymptomatic patients. The presence of outflow obstruction at baseline increases the risk of clinical deterioration.[81] The incidence of arrhythmias and syncope were not found to be increased during pregnancy.[82]
Management of HCM in pregnancy should focus on preventing blood loss and avoiding the use of drugs that cause vasodilatation.[11] Beta-blockers, diuretics, and calcium channel blockers should be used in patients with symptoms of elevated left ventricular filling pressure. Patients with history of syncope or life-threatening arrhythmias should be assessed for implantation of an automatic defibrillator.[80]
Vaginal delivery is preferred. Shortening of the second stage of labor by the use of forceps or vacuum assistance should be considered in patients with left ventricular outflow obstruction. Oxytocin is the preferred agent for induction as compared to prostaglandins due to the vasodilatory effect of the latter.
Peripartum cardiomyopathy
Peripartum cardiomyopathy is a rare disorder with incidence ranging between 1 in 1,485 live births to 1 in 15,000 live births.[83] Peripartum cardiomyopathy is defined as the development of heart failure in the last month of pregnancy or in the first 5 months after delivery without any identifiable etiology and with objective assessment of left ventricular dysfunction.[84] Risk factors associated with peripartum cardiomyopathy are maternal age older than 30 years,[85] gestational hypertension, and twin pregnancies.[86]
The association with gestational hypertension suggests a causal relationship; however, a study performed in women with preeclampsia revealed no change in left ventricular systolic function.[87] An autoimmune mechanism has been suggested on the basis of high titers of autoantibodies against human cardiac tissue proteins in the sera of patients with peripartum cardiomyopathy that are absent in patients with idiopathic cardiomyopathy.[88] More evidence supports myocarditis as the possible cause than other suggested etiologies.[84]
Therapy should follow general heart failure guidelines for pregnancy, keeping fetal safety in mind during pregnancy and breastfeeding. Angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers are contraindicated during pregnancy because of the risk of fetal renal agenesis.[7, 6]
Usual treatments rely on furosemide and nitrates or hydralazine. No reliable predictors exist for which of these patients may progress rapidly to need for heart transplant and which may substantively recover. During pregnancy, IV nitrates and/or hydralazine are often used; after delivery, ACE inhibitors may be initiated. Amlodipine has also been found to be beneficial in nonischemic cardiomyopathy[89] and may have anti-inflammatory effects,[90] adding extra benefit in peripartum cardiomyopathy.
Beta-receptor antagonists in dilated cardiomyopathy are safe and are not contraindicated in pregnancy, yet, due to the lack of studies in peripartum cardiomyopathy, initiation of this group of medications in the postpartum period seems to be a reasonable approach in patients who continue to have symptoms.[84]
Peripartum cardiomyopathy is associated with increased maternal and fetal risk. With improved therapy and awareness, the trend is toward better prognosis. A recent study reported an in-hospital mortality of 1.36%, with a total mortality of 2.1%,[91] which is a considerable improvement over previously reported mortality rates of 7-18%.[92, 93] The course of peripartum cardiomyopathy seems to differ from that of traditional cardiomyopathy with normalization of left ventricular dysfunction occurring in about 50% of patients within 6 months after delivery. Normalization of cardiac function was more likely in patients with left ventricular ejection fraction more than 30% at the time of diagnosis.[86]
An important clinical issue is the patient’s ability to have future pregnancies. Future pregnancies should be discouraged in patients who do not recover their left ventricular function. The risk of heart failure and death in women with persistently decreased left ventricular function may be as high as 20% with subsequent pregnancy.[94] Women with normalization of their left ventricular function (ejection fraction >50%) appear to have better outcomes than those with persistently depressed systolic function. Nevertheless, they do have a risk of heart failure symptoms and a significant drop in left ventricular ejection fraction with subsequent pregnancies.[94]
In patients with normalization of left ventricular function following delivery, subsequent pregnancies should be managed at high-risk centers. Coronary artery disease should be considered, particularly if a family history of early atherosclerotic disease exists, or other risk factors such as smoking, long-standing diabetes, dyslipidemia, or cocaine use.
PreviousNextCoronary Artery Disease in Pregnancy
Myocardial infarction complicating pregnancy is a rare occurrence, with an estimated incidence in the United States of 1 in 10,000 pregnancies.[95, 92] The risk of myocardial infarction is 3-4 fold higher in pregnancy when compared to nonpregnant reproductive age women,[96] and the incidence is expected to rise owing to increasing maternal age.[97]
Risk factors for coronary artery disease (CAD) in the childbearing age group include cigarette smoking, family history of premature CAD, an atherogenic lipid profile, diabetes mellitus, hypertension, preeclampsia, oral contraceptive use, and cocaine use.[98, 38]
These and other risk factors for CAD are discussed in the 2011 update to the American Heart Association guideline for the prevention of cardiovascular disease (CVD) in women.[10] Pregnancy contributes to these risk factors by increasing total cholesterol, low-density lipoprotein, and triglycerides, and decreasing high-density lipoproteins.[99, 100] Also, spontaneous coronary artery dissection and coronary spasm have been described more frequently as a cause for acute myocardial infarction in pregnant than in nonpregnant patients.
The diagnosis of myocardial infarction in pregnancy is established in the same way as in the nonpregnant state because clinical symptoms of infarction, EKG, and cardiac biomarkers (specifically troponin) are not routinely affected by pregnancy. Creatinine kinase and its MB fraction may be increased around the time of delivery.[96]
Treatment is also generally the same in pregnancy with consideration of fetal effects. Low-dose aspirin is considered safe during pregnancy. However, prolonged use of 100 mg aspirin can cause increased maternal bleeding complications and low birth weight.[101, 102] Beta-blockers are the drug of choice in pregnancy due to their safety profile, while nitrates and calcium channel blockers should be used with caution to avoid maternal hypotension.[11]
Thrombolytic therapy has limited data in pregnancy. No reports of teratogenic effects exist, but an increased risk of maternal hemorrhage exists.[103] The 2004 ACC/AHA guidelines consider pregnancy a relative contraindication to thrombolytic therapy.[104] Coronary reperfusion by percutaneous transluminal coronary angioplasty or coronary bypass graft surgery has been reported with favorable outcomes.[11, 105, 106]
The highest mortality in these cases has been in patients who have a myocardial infarction within the late third trimester.[107] This is likely due to the hemodynamic stress and cardiac decompensation that can occur in the peripartum period. If possible, delivery has been suggested to be delayed for at least 2-3 weeks after an acute MI.[13] Management during labor and delivery should focus on minimizing cardiac workload during delivery. Epidural anesthesia, medical management of hypertension, and possible invasive hemodynamic monitoring may be needed in labor. Vaginal delivery is still reasonable unless other obstetrical indications exist, although an assisted vaginal delivery is preferred to avoid a prolonged second stage.
Previous Contributor Information and DisclosuresAuthor
Tamam N Mohamad, MD Fellow, Department of Cardiology, Wayne State University, Detroit Medical Center
Tamam N Mohamad, MD is a member of the following medical societies: American College of Cardiology, American College of Physicians-American Society of Internal Medicine, American Medical Association, Michigan State Medical Society, and National Arab American Medical Association
Disclosure: Nothing to disclose.
Coauthor(s)
Hesham A Fakhri, MD Staff Physician, Department of Internal Medicine, Wayne State University, Detroit Receiving Hospital, Harper University Hospital, John D Dingell Veterans Affairs Medical Center
Hesham A Fakhri, MD is a member of the following medical societies: American College of Physicians, American Medical Association, and Michigan State Medical Society
Disclosure: Nothing to disclose.
Juan M Bernal, MD, MSc Interventional Cardiology Fellow, Massachusetts General Hospital, Boston, MA
Juan M Bernal, MD, MSc is a member of the following medical societies: American College of Cardiology, American College of Physicians, and Michigan State Medical Society
Disclosure: Nothing to disclose.
Deepak Thatai, MBBS, FACC Associate Professor, Department of Medicine, Wayne State University; Consulting Staff, Director of Cardiac Catheterization Lab, John D Dingell Veterans Affairs Medical Center
Deepak Thatai, MBBS, FACC is a member of the following medical societies: American College of Cardiology and American Heart Association
Disclosure: Nothing to disclose.
Erika Peterson, MD Assistant Professor, Department of Obstetrics and Gynecology, Section of Maternal-Fetal Medicine, Medical College of Wisconsin
Erika Peterson, MD is a member of the following medical societies: American College of Obstetricians and Gynecologists and Society for Maternal-Fetal Medicine
Disclosure: Nothing to disclose.
Specialty Editor Board
Justin D Pearlman, MD, ME, PhD, FACC, MA Chief, Division of Cardiology, Director of Cardiology Consultative Service, Director of Cardiology Clinic Service, Director of Cardiology Non-Invasive Laboratory, Director of Cardiology Quality Program KMC, Vice Chair of Medicine, UCLA
Justin D Pearlman, MD, ME, PhD, FACC, MA is a member of the following medical societies: American College of Cardiology, American College of Physicians, American Federation for Medical Research, International Society for Magnetic Resonance in Medicine, and Radiological Society of North America
Disclosure: Nothing to disclose.
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Salary Employment
David Chelmow, MD Leo J Dunn Distinguished Professor and Chair, Department of Obstetrics and Gynecology, Virginia Commonwealth University Medical Center
David Chelmow, MD is a member of the following medical societies: American College of Obstetricians and Gynecologists, American Medical Association, American Society for Colposcopy and Cervical Pathology, Association of Professors of Gynecology and Obstetrics, Council of University Chairs of Obstetrics and Gynecology, Phi Beta Kappa, Sigma Xi, Society for Gynecologic Investigation, and Society for Medical Decision Making
Disclosure: Nothing to disclose.
Chief Editor
Richard A Lange, MD Professor and Executive Vice Chairman, Department of Medicine, Director, Office of Educational Programs, University of Texas Health Science Center at San Antonio
Richard A Lange, MD is a member of the following medical societies: Alpha Omega Alpha, American College of Cardiology, American Heart Association, and Association of Subspecialty Professors
Disclosure: Nothing to disclose.
References
van Oppen AC, Stigter RH, Bruinse HW. Cardiac output in normal pregnancy: a critical review. Obstet Gynecol. Feb 1996;87(2):310-8. [Medline].
Robson SC, Hunter S, Moore M, et al. Haemodynamic changes during the puerperium: a Doppler and M-mode echocardiographic study. Br J Obstet Gynaecol. Nov 1987;94(11):1028-39. [Medline].
Shime J, Mocarski EJ, Hastings D, et al. Congenital heart disease in pregnancy: short- and long-term implications. Am J Obstet Gynecol. Feb 1987;156(2):313-22. [Medline].
Easterling TR, Benedetti TJ, Schmucker BC, et al. Maternal hemodynamics in normal and preeclamptic pregnancies: a longitudinal study. Obstet Gynecol. Dec 1990;76(6):1061-9. [Medline].
Robson SC, Dunlop W, Boys RJ, et al. Cardiac output during labour. Br Med J (Clin Res Ed). Nov 7 1987;295(6607):1169-72. [Medline].
Stout KK, Otto CM. Pregnancy in women with valvular heart disease. Heart. May 2007;93(5):552-8. [Medline].
Reimold SC, Rutherford JD. Clinical practice. Valvular heart disease in pregnancy. N Engl J Med. Jul 3 2003;349(1):52-9. [Medline].
Robson SC, Dunlop W, Moore M, et al. Combined Doppler and echocardiographic measurement of cardiac output: theory and application in pregnancy. Br J Obstet Gynaecol. Nov 1987;94(11):1014-27. [Medline].
Siu SC, Sermer M, Harrison DA, et al. Risk and predictors for pregnancy-related complications in women with heart disease. Circulation. 1997;96:2789-94.
[Guideline] Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the american heart association. Circulation. Mar 22 2011;123(11):1243-62. [Medline]. [Full Text].
U. Elkayam, Pregnancy and. cardiovascular disease. In: E. Braunwald, Editor. Heart Disease: A Textbook of Cardiovascular Medicine,. 107(1). Philadelphia: WB Saunders; (2005):pp. 1965-1981.
Mishra M, Chambers JB, Jackson G. Murmurs in pregnancy: an audit of echocardiography. BMJ. May 30 1992;304(6839):1413-4. [Medline].
Elkayam U, Gleicher N. Cardiac evaluation during pregnancy. In: Elkayam U, Gleicher N (eds). Cardiac Problems in Pregnancy. 3rd ed. New York: Wiley-Liss; 1998:pp 39-53.
Upshaw CB Jr. A study of maternal electrocardiograms recorded during labor and delivery. Am J Obstet Gynecol. May 1 1970;107(1):17-27. [Medline].
Ovando LA, Germiniani H, Miglino R, et al. [Maternal cardiac arrhythmias during labor and delivery]. Arq Bras Cardiol. Mar 1983;40(3):171-6. [Medline].
Colletti PM, Lee K. Cardiovascular imaging in the pregnant patient. In: Elkayam U, Gleicher N (eds). Cardiac Problems in Pregnancy. 3rd ed. New York: Wiley-Liss; 1998:pp 39-53.
ACC/AHA guidelines for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association. Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease). J Am Coll Cardiol. Nov 1998;32(5):1486-588. [Medline].
Soler-Soler J, Galve E. Worldwide perspective of valve disease. Heart. Jun 2000;83(6):721-5. [Medline].
Qasqas SA, McPherson C, Frishman WH, et al. Cardiovascular pharmacotherapeutic considerations during pregnancy and lactation. Cardiol Rev. Jul-Aug 2004;12(4):201-21. [Medline].
Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation: a reference guide to fetal and neonatal risk. Baltimore: Williams & Wilkins; 1998: xxii..
Sugrue D, Blake S, Troy P, et al. Antibiotic prophylaxis against infective endocarditis after normal delivery--is it necessary?. Br Heart J. Nov 1980;44(5):499-502. [Medline].
McFaul PB, Dornan JC, Lamki H, et al. Pregnancy complicated by maternal heart disease. A review of 519 women. Br J Obstet Gynaecol. Sep 1988;95(9):861-7. [Medline].
Petanovic M, Zagar Z. The significance of asymptomatic bacteremia for the newborn. Acta Obstet Gynecol Scand. Sep 2001;80(9):813-7. [Medline].
Furman B, Shoham-Vardi I, Bashiri A, et al. Clinical significance and outcome of preterm prelabor rupture of membranes: population-based study. Eur J Obstet Gynecol Reprod Biol. Oct 2000;92(2):209-16. [Medline].
Campuzano K, Roque H, Bolnick A, et al. Bacterial endocarditis complicating pregnancy: case report and systematic review of the literature. Arch Gynecol Obstet. Oct 2003;268(4):251-5. [Medline].
Hameed A, Karaalp IS, Tummala PP, et al. The effect of valvular heart disease on maternal and fetal outcome of pregnancy. J Am Coll Cardiol. Mar 1 2001;37(3):893-9. [Medline].
Silversides CK, Colman JM, Sermer M, et al. Cardiac risk in pregnant women with rheumatic mitral stenosis. Am J Cardiol. Jun 1 2003;91(11):1382-5. [Medline].
Clark SL. Cardiac disease in pregnancy. Crit Care Clin. Oct 1991;7(4):777-97. [Medline].
Lesniak-Sobelga A, Tracz W, KostKiewicz M, et al. Clinical and echocardiographic assessment of pregnant women with valvular heart diseases--maternal and fetal outcome. Int J Cardiol. Mar 2004;94(1):15-23. [Medline].
al Kasab SM, Sabag T, al Zaibag M, et al. Beta-adrenergic receptor blockade in the management of pregnant women with mitral stenosis. Am J Obstet Gynecol. Jul 1990;163(1 Pt 1):37-40. [Medline].
Hurst AK, Hoffman K, Frishman WH, et al. The use of B-adrenergic blocking agents in pregnancy and lactation. In: Elkayam U, Gleicher N, editors. Cardiac Problems in Pregnancy. New York, NY: Wiley- Liss; 1998:351-90.
Cohen F, Garty M. Diuretics in pregnancy. In: Elkayam U, Gleicher N, editors. Cardiac Problems in Pregnancy. New York, NY: Wiley- Liss; 1998:351- 8.
Allen NM, Page RL. Procainamide administration during pregnancy. Clin Pharm. Jan 1993;12(1):58-60. [Medline].
Dekaban AS. Abnormalities in children exposed to x-radiation during various stages of gestation: tentative timetable of radiation injury to the human fetus. I. J Nucl Med. Sep 1968;9(9):471-7. [Medline].
Weiss BM, von Segesser LK, Alon E, et al. Outcome of cardiovascular surgery and pregnancy: a systematic review of the period 1984-1996. Am J Obstet Gynecol. Dec 1998;179(6 Pt 1):1643-53. [Medline].
Kuczkowski KM, van Zundert A. Anesthesia for pregnant women with valvular heart disease:the state-of-the-art. J Anesth. 2007;21:252-257.
Bhatla N, Lal S, Behera G, et al. Cardiac disease in pregnancy. Int J Gynaecol Obstet. Aug 2003;82(2):153-9. [Medline].
Hameed AB, Tummala PP, Goodwin TM, et al. Unstable angina during pregnancy in two patients with premature coronary atherosclerosis and aortic stenosis in association with familial hypercholesterolemia. Am J Obstet Gynecol. May 2000;182(5):1152-5. [Medline].
Bhargava B, Agarwal R, Yadav R, et al. Percutaneous balloon aortic valvuloplasty during pregnancy: use of the Inoue balloon and the physiologic antegrade approach. Cathet Cardiovasc Diagn. Dec 1998;45(4):422-5. [Medline].
Tumelero RT, Duda NT, Tognon AP, et al. Percutaneous balloon aortic valvuloplasty in a pregnant adolescent. Arq Bras Cardiol. Jan 2004;82(1):98-101, 94-7. [Medline].
Drenthen W, Pieper PG, Roos-Hesselink JW, et al. Outcome of Pregnancy in woman with congenital heart disease. J Am Coll Cardiol. 2007;49:2303.
Yap SC, Drenthen W, Pieper PG, et al. Risk of complications during pregnancy in women with congenital aortic stenosis. Inter J of Card. 2008;126:240-246.
Jamieson WR, Miller DC, Akins CW, et al. Pregnancy and bioprostheses: influence on structural valve deterioration. Ann Thorac Surg. Aug 1995;60(2 Suppl):S282-6; discussion S287. [Medline].
Salazar E, Espinola N, Roman L, et al. Effect of pregnancy on the duration of bovine pericardial bioprostheses. Am Heart J. Apr 1999;137(4 Pt 1):714-20. [Medline].
Sadler L, McCowen L, White H et al. Pregnancy outcomes and cardiac complication in women with mechanical, bioprosthetic and homograft valves. BJOG. 2000;107:245.
Bonow RO, Carabello BA, Chatterjee K et al. ACC/AHA 2006 Guidelines for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): Developed in Collaboration With the Society of Cardiovascular Anesthesiologists : Endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84-e231.
Bates SM, Greer IA, Pabinger I, et al. Venous thromboembolism, thrombophilia, antithrombotic therapy and pregnancy: American College of Chest Physicians Evidence Based Clinical Practice Guidelines. Chest. 2008;133:844S.
Shapira Y, Sagie A, Battler. Low-molecular weight heparin for the treatment of patients with mechanical heart valves. Clin Cardiol. 2002;25:323.
Quinn J, Von Klemperer K, Brooks R, Peebles D, Walker F, Cohen H. Use of high intensity adjusted dose low moleclar weight heparin in women with mechanical heart valves during pregnancy: a single center experience. Haematologica. Nov 2009;94:1608-12.
Nieminen HP, Jokinen EV, Sairanen HI. Late results of pediatric cardiac surgery in Finland: a population-based study with 96% follow-up. Circulation. Jul 31 2001;104(5):570-5. [Medline].
Thorne S, Deanfield J. Long-term outlook in treated congenital heart disease. Arch Dis Child. Jul 1996;75(1):6-8. [Medline].
Stout K. Pregnancy in women with congenital heart disease: the importance of evaluation and counselling. Heart. Jun 2005;91(6):713-4. [Medline].
Daliento L, Somerville J, Presbitero P, et al. Eisenmenger syndrome. Factors relating to deterioration and death. Eur Heart J. Dec 1998;19(12):1845-55. [Medline].
Presbitero P, Somerville J, Stone S, et al. Pregnancy in cyanotic congenital heart disease. Outcome of mother and fetus. Circulation. Jun 1994;89(6):2673-6. [Medline].
Avila WS, Rossi EG, Ramires JA, et al. Pregnancy in patients with heart disease: experience with 1,000 cases. Clin Cardiol. Mar 2003;26(3):135-42. [Medline].
Meijer JM, Pieper PG, Drenthen W, et al. Pregnancy, fertility, and recurrence risk in corrected tetralogy of Fallot. Heart. Jun 2005;91(6):801-5. [Medline].
Steer PJ. Pregnancy and contraception. Gatzoulis MA, Swan L, Therrien J, Pantely GA, eds. Adult congenital heart disease: A practical guide. Oxford: BMJ Publishing, Blackwell Publishing,; 2005:16-35.
Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA. Jun 11 1997;277(22):1794-801. [Medline].
Veldtman GR, Connolly HM, Grogan M, et al. Outcomes of pregnancy in women with tetralogy of Fallot. J Am Coll Cardiol. Jul 7 2004;44(1):174-80. [Medline].
Actis Dato GM, Rinaudo P, Revelli A, et al. Atrial septal defect and pregnancy: a retrospective analysis of obstetrical outcome before and after surgical correction. Minerva Cardioangiol. Mar 1998;46(3):63-8. [Medline].
Warnes CA, Elkayam U. Congenital heart disease in pregnancy. In: Elkayam U, Gleicher N, editors. Cardiac Problems in Pregnancy. 3rd ed. New York, NY: Wiley-Liss; 1998:39 -55.
Siu SC, Colman JM. Heart disease and pregnancy. Heart. Jun 2001;85(6):710-5. [Medline].
Siu SC, Sermer M, Harrison DA, et al. Risk and predictors for pregnancy-related complications in women with heart disease. Circulation. Nov 4 1997;96(9):2789-94. [Medline].
Actis Dato GM, Cavaglia M, Aidala E, et al. Patent ductus arteriosus. Follow-up of 677 operated cases 40 years later. Minerva Cardioangiol. Jul-Aug 1999;47(7-8):245-54. [Medline].
Saidi AS, Bezold LI, Altman CA, et al. Outcome of pregnancy following intervention for coarctation of the aorta. Am J Cardiol. Sep 15 1998;82(6):786-8. [Medline].
Beauchesne LM, Connolly HM, Ammash NM, et al. Coarctation of the aorta: outcome of pregnancy. J Am Coll Cardiol. Nov 15 2001;38(6):1728-33. [Medline].
Plunkett MD, Bond LM, Geiss DM. Staged repair of acute type I aortic dissection and coarctation in pregnancy. Ann Thorac Surg. Jun 2000;69(6):1945-7. [Medline].
Beauchesne LM, Connolly HM, Ammash NM, Warnes CA. Coarctation of the aorta: outcome of pregnancy. J Am Coll Cardiol. Nov/2001;38:1728-33. [Medline].
Anderson RA, Fineron PW. Aortic dissection in pregnancy: Importance of pregnancy-induced changes in the vessel wall and bicuspid aortic valve in pathogenesis. Br J Obstet Gynaecol. 1994;101:1085.
Therrien J, Marx GR, Gatzoulis MA. Late problems in tetralogy of Fallot--recognition, management, and prevention. Cardiol Clin. Aug 2002;20(3):395-404. [Medline].
Clarkson PM, Wilson NJ, Neutze JM, North RA, Calder AL, Barratt-Boyes BG. Outcome of pregnancy after the Mustard operation for transposition of the great arteries with intact ventricular septum. J Am Coll Cardiol. July/1994;24:190-3. [Medline].
Lao TT, Sermer M, Colman JM. Pregnancy after the Fontan procedure for tricuspid atresia. A case report. J Reprod Med. Apr 1996;41:287-90. [Medline].
Nitsche JF, Phillips SD, Rose CH, Brost BC, Watson WJ. Pregnancy and delivery in patients with Fontan circulation. A case report and review of obstetric management. Obstet Gynocol Survey. 2009;64:607-14.
Drenthen W, Pieper PG, Roos-Hesselink JW et al. Pregnancy and delivery in women after Fontan palliation. Heart. 2006;92:1290-94.
Drenthen W, Pieper PG, Roos- Hesselink JW et al. Outcome of pregnancy in women with congenital heart disease: a literature review. J Am Coll Cardiol. 2007;49:2303.
Walker F. Pregnancy and teh various forms of the Fontan circulation. Heart. 2007;93:152-54.
Weiss BM, Hess O. Perioperative cardiovascular evaluation for noncardiac surgery: congenital heart diseases and heart diseases in pregnancy deserve better guidelines. Circulation. Jan 21 1997;95(2):530-1. [Medline].
Somerville J. The Denolin Lecture: The woman with congenital heart disease. Eur Heart J. Dec 1998;19(12):1766-75. [Medline].
Weiss BM, Hess OM. Pulmonary vascular disease and pregnancy: current controversies, management strategies, and perspectives. Eur Heart J. Jan 2000;21(2):104-15. [Medline].
Elkayam U, Dave R. Hypertrophic cardiomyopathy and pregnancy. In: Elkayam U, Gleicher N (eds). Cardiac Problems in Pregnancy. 3rd ed. New York: Wiley-Liss; 1998:pp 211-221.
Thaman R, Varnava A, Hamid MS, et al. Pregnancy related complications in women with hypertrophic cardiomyopathy. Heart. Jul 2003;89(7):752-6. [Medline].
Thaman R, Varnava A, Hamid MS, et al. Pregnancy related complications in women with hypertrophic cardiomyopathy. Heart. Jul 2003;89(7):752-6. [Medline].
Whitehead SJ, Berg CJ, Chang J. Pregnancy-related mortality due to cardiomyopathy: United States, 1991-1997. Obstet Gynecol. Dec 2003;102(6):1326-31. [Medline].
Pearson GD, Veille JC, Rahimtoola S, et al. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA. Mar 1 2000;283(9):1183-8. [Medline].
Demakis JG, Rahimtoola SH, Sutton GC, et al. Natural course of peripartum cardiomyopathy. Circulation. Dec 1971;44(6):1053-61. [Medline].
Elkayam U, Akhter MW, Singh H, et al. Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation. Apr 26 2005;111(16):2050-5. [Medline].
Borghi C, Esposti DD, Immordino V, et al. Relationship of systemic hemodynamics, left ventricular structure and function, and plasma natriuretic peptide concentrations during pregnancy complicated by preeclampsia. Am J Obstet Gynecol. Jul 2000;183(1):140-7. [Medline].
Ansari AA, Neckelmann N, Wang YC, et al. Immunologic dialogue between cardiac myocytes, endothelial cells, and mononuclear cells. Clin Immunol Immunopathol. Aug 1993;68(2):208-14. [Medline].
Packer M, O'Connor CM, Ghali JK, et al. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. Prospective Randomized Amlodipine Survival Evaluation Study Group. N Engl J Med. Oct 10 1996;335(15):1107-14. [Medline].
Mohler ER 3rd, Sorensen LC, Ghali JK, et al. Role of cytokines in the mechanism of action of amlodipine: the PRAISE Heart Failure Trial. Prospective Randomized Amlodipine Survival Evaluation. J Am Coll Cardiol. Jul 1997;30(1):35-41. [Medline].
Mielniczuk LM, Williams K, Davis DR, et al. Frequency of peripartum cardiomyopathy. Am J Cardiol. Jun 15 2006;97(12):1765-8. [Medline].
Hands ME, Johnson MD, Saltzman DH, et al. The cardiac, obstetric, and anesthetic management of pregnancy complicated by acute myocardial infarction. J Clin Anesth. Jul-Aug 1990;2(4):258-68. [Medline].
Witlin AG, Mabie WC, Sibai BM. Peripartum cardiomyopathy: an ominous diagnosis. Am J Obstet Gynecol. Jan 1997;176(1 Pt 1):182-8. [Medline].
Elkayam U;Tummala PP;Rao K; Akhter MW karaalp IS; Wani OR; Hameed A; Gviazda I; Shotan A. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med. May 2001;344:1567-71. [Medline].
Hankins GD, Wendel GD Jr, Leveno KJ, et al. Myocardial infarction during pregnancy: a review. Obstet Gynecol. Jan 1985;65(1):139-46. [Medline].
Roth A, Elkayam U. Acute Myocardial Infarction Associated with Pregnancy. J Am Coll Cardiol. 2008;52:171-180.
Paulson RJ, Boostanfar R, Saadat P, et al. Pregnancy in the sixth decade of life: obstetric outcomes in women of advanced reproductive age. JAMA. Nov 13 2002;288(18):2320-3. [Medline].
Roth A, Elkayam U. Acute myocardial infarction and pregnancy. In: Elkayam U, Gleicher N 9eds0. Cardiac Problems in Pregnancy. 3rd ed. New York: Wiley-Liss; 1998,:pp 121-130.
Brizzi P, Tonolo G, Esposito F, et al. Lipoprotein metabolism during normal pregnancy. Am J Obstet Gynecol. Aug 1999;181(2):430-4. [Medline].
Gunderson EP, Lewis CE, Murtaugh MA, et al. Long-term plasma lipid changes associated with a first birth: the Coronary Artery Risk Development in Young Adults study. Am J Epidemiol. Jun 1 2004;159(11):1028-39. [Medline].
Caritis S, Sibai B, Hauth J, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. Mar 12 1998;338(11):701-5. [Medline].
Subtil D, Goeusse P, Puech F, et al. Aspirin (100 mg) used for prevention of pre-eclampsia in nulliparous women: the Essai Régional Aspirine Mère-Enfant study (Part 1). BJOG. May 2003;110(5):475-84. [Medline].
Turrentine MA, Braems G, Ramirez MM. Use of thrombolytics for the treatment of thromboembolic disease during pregnancy. Obstet Gynecol Surv. July 1995;50:534-41.
Antman EM, ANBE dt, Armstrrong PW et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction. Available at http://www.acc.org/qualityandscience/clinical/statements.htm. Accessed July 26th 2010.
Dwyer BK, Taylor L, Fuller A, Brummel C, Lyell DJ. Percutaneous transluminal coronary angioplasty and stent placement in pregnancy. Obstet Gynecol. Nov 2005;106(5 Pt 2):1162-4. [Medline].
Boztosum B, Oleay A, Avei A, Kirma C. Treatment of acute myocardial infarction in pregnancy with coronary artery balloon angioplasty and stenting: Use of tirofiban and clopidogrel. Int J Cardiol. 2008;127:413-16.
Ladner HE, Danielsen B, Gilbert WM. Acute Myocardial Infarction in Preganncy and the Puerperium: A population based study. Am J Obstet Gynecol. March 2005;105:480-84.
ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. Jan 15 2003;167(2):211-77. [Medline].
Bernal JM, Miralles PJ. Cardiac surgery with cardiopulmonary bypass during pregnancy. Obstet Gynecol Surv. Jan 1986;41(1):1-6. [Medline].
Bozkurt B, Villaneuva FS, Holubkov R, et al. Intravenous immune globulin in the therapy of peripartum cardiomyopathy. J Am Coll Cardiol. Jul 1999;34(1):177-80. [Medline].
Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation. 6th edition. Baltimore, MD: Lippincott Williams and Wilkins; 2002:421,1461.
Chen FG, Koh KF, Chong YS. Cardiac arrest associated with sulprostone use during caesarean section. Anaesth Intensive Care. Jun 1998;26(, Cardiovascular Disease and Pregnancy
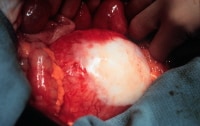 Aneurysm with retroperitoneal fibrosis and adhesion of the duodenum and fibrosis.
Aneurysm with retroperitoneal fibrosis and adhesion of the duodenum and fibrosis. 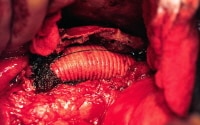 Endoaneurysmorrhaphy.
Endoaneurysmorrhaphy.  Endovascular grafts. NextEpidemiologyUnited States statistics
Endovascular grafts. NextEpidemiologyUnited States statistics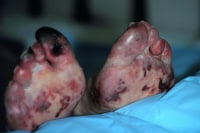 Atheroemboli from small abdominal aortic aneurysms produce livedo reticularis of the feet (ie, blue toe syndrome). Aortocaval fistulae
Atheroemboli from small abdominal aortic aneurysms produce livedo reticularis of the feet (ie, blue toe syndrome). Aortocaval fistulae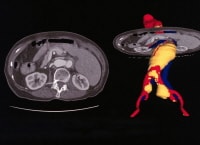 Enhanced spiral CT scans with multiplanar reconstruction and a CT angiogram.
Enhanced spiral CT scans with multiplanar reconstruction and a CT angiogram. 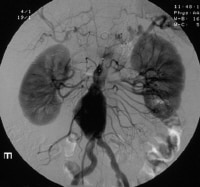 Angiography is used to diagnose the renal area. In this instance, an endoleak represented continued pressurization of the sac. Echocardiography
Angiography is used to diagnose the renal area. In this instance, an endoleak represented continued pressurization of the sac. Echocardiography




