Vertebral artery dissection (VAD) is a relatively rare but increasingly recognized cause of stroke in patients younger than 45 years. Although the term spontaneous VAD is used to describe cases that do not involve significant blunt or penetrating trauma as a precipitating factor, many patients with so-called spontaneous VAD have a history of trivial or minor injury involving some degree of cervical distortion.
Essential update: Long-term benefit from endovascular therapy for vertebral artery dissectionIn a study of 73 patients treated for VAD with endovascular internal trapping, stable and durable results were demonstrated over a mean follow-up of 55.6 months. Recanalization was rare and observed only in 2 patients with ruptured VAD, both within 3 months after initial treatment without rupture. Cranial nerve paresis was observed in 8.21% of patients, perforating ischemia was seen in 9.59%, and spinal cord infarction was seen in 2.74%. Patient ratings of quality of life were good.[6]
Signs and symptomsThe typical patient with VAD is a young person who experiences severe occipital headache and posterior nuchal pain following a head or neck injury and subsequently develops focal neurologic signs attributable to ischemia of the brainstem or cerebellum. The focal signs may not appear until after a latent period lasting as long as 3 days, however, and delays of weeks and years also have been reported. Many patients present only at the onset of neurologic symptoms.
When neurologic dysfunction does occur, patients most commonly report symptoms attributable to lateral medullary dysfunction (ie, Wallenberg syndrome). Patient history may include the following:
Ipsilateral facial dysesthesia (pain and numbness)[7] - Most common symptom Dysarthria or hoarseness (cranial nerves [CN] IX and X)Contralateral loss of pain and temperature sensation in the trunk and limbsIpsilateral loss of taste (nucleus and tractus solitarius)HiccupsVertigo[7] Nausea and vomitingDiplopia or oscillopsia (image movement experienced with head motion)Dysphagia (CN IX and X)DisequilibriumUnilateral hearing loss[8]Rarely, patients may manifest the following symptoms of a medial medullary syndrome:
Contralateral weakness or paralysis (pyramidal tract)Contralateral numbness (medial lemniscus)Depending upon which areas of the brainstem or cerebellum are experiencing ischemia, the following signs may be present:
Limb or truncal ataxiaNystagmus[9] Ipsilateral Horner syndrome[4] Ipsilateral hypogeusia or ageusia (ie, diminished or absent sense of taste)Ipsilateral impairment of fine touch and proprioceptionContralateral impairment of pain and thermal sensation in the extremities (ie, spinothalamic tract)Lateral medullary syndrome[10]Cerebellar findings may include the following:
NystagmusMedial medullary syndromeTongue deviation to the side of the lesion (impairment of CN XII)Contralateral hemiparesisIpsilateral impairment of fine touch and proprioception (nucleus gracilis)Internuclear ophthalmoplegia (lesion of the medial longitudinal fasciculus)See Clinical Presentation for more detail.
DiagnosisImaging studies in patients with suspected VAD may include the following:
Computed tomography – Identifies subarachnoid hemorrhage[9] Four-vessel cerebral angiography[11] – Once the criterion standard for diagnosis, now largely supplanted by noninvasive techniques Magnetic resonance imaging[5, 12, 11, 13, 14] – Detects both the intramural thrombus and intimal flap that are characteristic of VAD[11] ; hyperintensity of the vessel wall seen on T1-weighted axial images is considered by some to be pathognomonic of VAD Magnetic resonance angiography[5, 12, 15, 13, 14] – Can identify a pseudolumen and aneurysmal dilation of the artery[11] Vascular duplex scanning – Demonstrates abnormal flow in 95% of patients with VAD,[5] but shows signs specific to VAD (eg, segmental dilation of the vessel, eccentric channel) in only 20% Transcranial Doppler – Approximately 75% sensitive to the flow abnormalities seen in VAD; useful also in detecting high-intensity signals (HITS), which are characteristic of microemboli propagating distally as a result of the dissectionBecause VAD occurs in young, generally healthy individuals, laboratory evaluation is directed toward establishing baseline parameters in anticipation of anticoagulant therapy, as follows:
Prothrombin time (PT) with international normalized ratio (INR)Activated partial thromboplastin time (aPTT)In addition, elevation of the erythrocyte sedimentation rate (ESR) may suggest vasculitis involving the cerebrovascular circulation.
See Workup for more detail.
ManagementAcute management of proven or suspected spontaneous VAD is as follows[16] :
Anticoagulants and antiplatelet agents are the drugs of choice to prevent thromboembolic disordersMore potent agents (eg, intra-arterial thrombolytics) have been used in selected casesSee Treatment and Medication for more detail.
Image library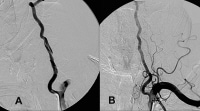 A, Dissection of the left vertebral artery secondary to guidewire injury. B, Complete resolution occurred in 6 months with only aspirin and clopidogrel (Plavix; Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership, Bridgewater, NJ) therapy. NextBackground
A, Dissection of the left vertebral artery secondary to guidewire injury. B, Complete resolution occurred in 6 months with only aspirin and clopidogrel (Plavix; Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership, Bridgewater, NJ) therapy. NextBackgroundVertebral artery dissection (VAD) is an increasingly recognized cause of stroke in patients younger than 45 years.[1, 2, 3, 4] Although its pathophysiology and treatment closely resemble that of its sister condition, carotid artery dissection (CAD), the clinical presentation, etiology, and epidemiological profile of VADs are unique. In particular, advances in imaging have contributed to growing awareness of this entity.[5]
PreviousNextPathophysiologyAn expanding hematoma in the vessel wall is the root lesion in VAD. This intramural hematoma can arise spontaneously or as a secondary result of minor trauma, through hemorrhage of the vasa vasorum within the media of the vessel. It also can be introduced through an intimal flap that develops at the level of the inner lumen of the vessel. Major trauma is also an increasingly recognized cause of VAD.[17]
This intramural hemorrhage can evolve in a variety of ways, resulting in any of the following consequences:
The hematoma may seal off and, if sufficiently small, remain largely asymptomatic.If the dissection is subintimal, the expanding hematoma may partially or completely occlude the vertebral artery or one of its branches. Extensive dissections (those that extend intracranially and involve the basilar artery) result in infarctions of the brainstem, cerebellum or, rarely, the spinal cord. Subintimal dissections also may rupture back into the vertebral artery, thus creating a false lumen (pseudolumen). Subadventitial dissections tend to cause pseudoaneurysmal dilation of the vertebral artery, which may compress adjacent neurologic structures. These subadventitial dissections are prone to rupture through the adventitia, resulting in subarachnoid hemorrhage. In an autopsy series of more than 100 patients with subarachnoid hemorrhage, 5% of the hemorrhages were deemed the result of VAD. The intimal disruption and low flow states that arise in VAD create a thrombogenic milieu in which emboli may form and propagate distally. This results in transient ischemia or infarction.An understanding of the anatomy of the vertebral artery is helpful. The course of the vertebral artery usually is divided into 4 sections as follows:
Segment I runs from its takeoff at the first branch of the subclavian artery to the transverse foramina of cervical vertebra C5 or C6. Segment II runs entirely within the transverse foramina of C5/C6 to C2.Segment III, a tortuous segment, begins at the transverse foramen of C2, runs posterolaterally to loop around the posterior arch of C1, and passes subsequently between the atlas and the occiput. This segment is encased in muscles, nerves, and the atlanto-occipital membrane. Segment IV, the intracranial segment, begins as it pierces the dura at the foramen magnum and continues until the junction of the pons and medulla, where the vertebral arteries merge to join the larger proximal basilar trunk.Spontaneous dissection of the vertebral artery usually occurs in the tortuous distal extracranial segment (segment III) but may extend into the intracranial portion or segment IV.
PreviousNextEpidemiologyFrequencyUnited StatesDissections of the extracranial cervical arteries are relatively rare. The combined incidence of both VAD and CAD is estimated to be 2.6 per 100,000. However, cervical dissections are the underlying etiology in as many as 20% of the ischemic strokes presenting in younger patients aged 30-45 years. Among all extracranial cervical artery dissections, CAD is 3-5 times more common than VAD.[7]
Mortality/MorbidityVertebral artery dissection (VAD) has been associated with a 10% mortality rate in the acute phase. Death is the result of extensive intracranial dissection, brainstem infarction, or subarachnoid hemorrhage.[10] Those who survive the initial crisis do remarkably well, with long-term sequelae rare.SexThe female-to-male ratio is 3:1.
AgeIn contrast to atherothrombotic disease of the vertebrobasilar circulation, VAD occurs in a much younger population. The average age is 40 years; the average age of a patient with CAD is closer to 47 years.[12]
PreviousProceed to Clinical Presentation , Vertebral Artery Dissection





0 comments:
Post a Comment