Atrial fibrillation (AF) has strong associations with other cardiovascular diseases, such as heart failure, coronary artery disease (CAD), valvular heart disease, diabetes mellitus, and hypertension. It is characterized by an irregular and often rapid heartbeat. The exact mechanisms by which cardiovascular risk factors predispose to AF are not understood fully but are under intense investigation. Catecholamine excess, hemodynamic stress, atrial ischemia, atrial inflammation, metabolic stress, and neurohumoral cascade activation are all purported to promote AF.
Essential update: Bleeding complications less severe with dabigatranIn a meta-analysis of pooled patient-level data on more than 1000 patients who suffered major bleeding, this complication was generally less critical and more manageable in patients being treated with dabigatran than in those on warfarin therapy. For instance, in patients treated with dabigatran, the worst major bleeds tended to be gastrointestinal, while in those treated with warfarin, most of the worst bleeds were intracranial and therefore more difficult to treat.[1, 2]
The study's authors, Majeed et al, used data from the AF trial RE-LY and the venous thromboembolism – prevention trials RECOVER, RECOVER II, RE-MEDY, and RESONATE.
Compared with patients receiving warfarin, dabigatran-treated patients who experienced a major bleeding event tended to be older, with worse renal function, and were more often being treated concomitantly with a nonsteroidal anti-inflammatory agent, such as aspirin. More red blood cell transfusions, but less plasma, were required by dabigatran patients with major bleeds. These patients also spent less time in intensive care and had a lower mortality rate than the warfarin patients.
Signs and symptomsThe clinical presentation of AF spans the entire spectrum from asymptomatic AF with rapid ventricular response to cardiogenic shock or devastating cerebrovascular accident (CVA). Unstable patients requiring immediate direct current (DC) cardioversion include the following:
Patients with decompensated congestive heart failure (CHF)Patients with hypotensionPatients with uncontrolled angina/ischemiaInitial history and physical examination include the following:
Documentation of clinical type of AF (paroxysmal, persistent, or permanent)Assessment of type, duration, and frequency of symptomsAssessment of precipitating factors (eg, exertion, sleep, caffeine, alcohol use)Assessment of modes of termination (eg, vagal maneuvers)Documentation of prior use of antiarrhythmics and rate-controlling agentsAssessment of presence of underlying heart diseaseDocumentation of any previous surgical or percutaneous AF ablation proceduresAirway, breathing, and circulation (ABCs)Vital signs (particularly heart rate, blood pressure, respiratory rate, and oxygen saturation)Evaluation of head and neck, lungs, heart, abdomen, lower extremities, and nervous systemSee Clinical Presentation for more detail.
DiagnosisFindings from 12-lead electrocardiography (ECG) usually confirm the diagnosis of AF and include the following:
Typically irregular ventricular rateAbsence of discrete P waves, replaced by irregular, chaotic F waves, in the setting of irregular QRS complexesAberrantly conducted beats after long-short R-R cycles (ie, Ashman phenomenon)Heart rate (typically 110-140 beats/min, rarely >160-170 beats/min)PreexcitationLeft ventricular hypertrophyBundle-branch blockAcute or prior myocardial infarction (MI)Transthoracic echocardiography (TTE) is helpful for the following applications:
To evaluate for valvular heart diseaseTo evaluate atrial and ventricular chamber and wall dimensionsTo estimate ventricular function and evaluate for ventricular thrombiTo estimate pulmonary systolic pressure (pulmonary hypertension)To evaluate for pericardial diseaseTransesophageal echocardiography (TEE) is helpful for the following applications:
To evaluate for left atrial thrombus (particularly in the left atrial appendage)To guide cardioversion (if thrombus is seen, cardioversion should be delayed)See Workup for more detail.
ManagementThe cornerstones of AF management are rate control and anticoagulation,[3] as well as rhythm control for those symptomatically limited by AF. The clinical decision to use a rhythm-control or a rate-control strategy requires integrated consideration of the following:
Degree of symptomsLikelihood of successful cardioversionPresence of comorbiditiesCandidacy for AF ablationThe 2006 American College of Cardiology (ACC)/American Heart Association (AHA)/European Society of Cardiology (ESC) guidelines on anticoagulation for patients with nonvalvular AF include the following[4] :
No risk factors: Aspirin 81-325 mg/day1 moderate risk factor: Aspirin 81-325 mg/day or warfarin (international normalized ratio [INR] 2-3)Any high-risk factor or >1 moderate-risk factor: Warfarin (INR 2-3)Risk factors are as follows:
High-risk factors: Prior stroke or transient ischemic attack (TIA), systemic thromboembolismModerate-risk factors: Age >75 years, hypertension, heart failure, left ventricular function Risk factors of unknown significance: Female sex, age 65-74 years, coronary artery disease, thyrotoxicosisNew-onset AF:
ACC/AHA/ESC 2006 guidelines for new-onset AF include the following[4] :
An initial rate-control strategy is “reasonable†for asymptomatic or minimally symptomatic older patients with hypertension and comorbid cardiovascular disease For younger individuals, especially those without significant comorbid cardiovascular disease, an initial rhythm-control strategy may be betterAgents used for rate control in new-onset AF include the following:
DiltiazemMetoprololDigoxin (rarely as monotherapy)Amiodarone (mainly for patients who are intolerant of or unresponsive to other agents)Anticoagulation is indicated as follows:
Patients with newly diagnosed AF and those awaiting electrical cardioversion can be started on intravenous (IV) heparin or low-molecular-weight heparin (LMWH) Concomitantly, patients can be started on warfarin in an inpatient setting while awaiting a therapeutic INR value (2-3)Oral direct thrombin inhibitors may present an alternative to warfarin in a higher-risk population with nonvalvular AFNewer oral anticoagulants that have been approved by the US Food and Drug Administration (FDA) and may be considered as alternatives to warfarin include the following:
Dabigatran (direct thrombin inhibitor)Rivaroxaban (highly selective direct factor Xa inhibitor)Apixaban (factor Xa inhibitor)Long-term management of AF:
Optimal long-term strategies for AF management should be based on a thoroughly integrated consideration of patient-specific factors and likelihood of success. Selection of an appropriate antithrombotic regimen should be balanced between the risk of stroke and the risk of bleeding. Factors that increase the risk of bleeding with warfarin therapy include the following:
History of bleeding (the strongest predictive risk factor)Age older than 75 yearsLiver or renal diseaseMalignancyThrombocytopenia or aspirin useHypertensionDiabetes mellitusAnemiaPrior strokeFall riskGenetic predispositionSupratherapeutic INRAlternatives to warfarin:
If warfarin will not be used, adding clopidogrel to aspirin may be considered[5] Updated ACC/AHA/Heart Rhythm Society (HRS) guidelines on AF include a class Ib recommendation for dabigatran[6] for preventing stroke and systemic thromboembolism in patients with paroxysmal-to-permanent atrial fibrillation and risk factors for stroke or systemic embolizationAgents used for rate control include the following:
Oral beta-blockersNondihydropyridine calcium channel blockersDigoxinAmiodaroneAgents used for rhythm control include the following:
FlecainidePropafenoneDofetilideAmiodaroneDronedaroneSotalolCatheter ablation performed in experienced centers is recommended in the 2011 update to the ACCF/AHA/HRS AF guidelines for the following indications[5] :
It is recommended as an alternative to pharmacologic therapy to prevent recurrent paroxysmal AF in significantly symptomatic patients with little or no structural heart disease or severe pulmonary disease[7] It is reasonable as a treatment for symptomatic persistent AFIt may be reasonable as a treatment for symptomatic paroxysmal AF in patients with some structural heart diseaseSee Treatment and Medication for more detail.
Image library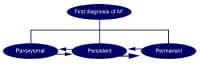 Classification scheme for patients with atrial fibrillation. NextBackground
Classification scheme for patients with atrial fibrillation. NextBackgroundClassification of atrial fibrillation (AF) begins with distinguishing a first detectable episode, irrespective of whether it is symptomatic or self-limited. Published guidelines from an American College of Cardiology (ACC)/American Heart Association (AHA)/European Society of Cardiology (ESC) committee of experts on the treatment of patients with atrial fibrillation recommend classification of AF into the following 3 patterns (also see the image below)[8] :
Paroxysmal AF – Episodes of AF that terminate spontaneously within 7 days (most episodes last less than 24 hours)Persistent AF - Episodes of AF that last more than 7 days and may require either pharmacologic or electrical intervention to terminate Permanent AF - AF that has persisted for more than 1 year, either because cardioversion has failed or because cardioversion has not been attempted Classification scheme for patients with atrial fibrillation.
Classification scheme for patients with atrial fibrillation. This classification schema pertains to cases that are not related to a reversible cause of AF (eg, thyrotoxicosis, electrolyte abnormalities, acute ethanol intoxication). Atrial fibrillation secondary to acute myocardial infarction, cardiac surgery, pericarditis, pulmonary embolism, or acute pulmonary disease is considered separately because, in these situations, AF is less likely to recur once the precipitating condition has been treated adequately and has resolved.
Paroxysmal AFAtrial fibrillation is considered to be recurrent when a patient has 2 or more episodes. If recurrent AF terminates spontaneously, it is designated as paroxysmal.
Some patients with paroxysmal AF, typically younger patients, have been found to have distinct electrically active foci within their pulmonary veins. These patients generally have many atrial premature beats noted on Holter monitoring. Isolation or elimination of these foci can lead to elimination of the trigger for paroxysms of AF.
Paroxysmal AF may progress to permanent AF, and aggressive attempts to restore and maintain sinus rhythm may prevent comorbidities associated with AF.
Persistent AFIf recurrent AF is sustained, it is considered persistent, irrespective of whether the arrhythmia is terminated by either pharmacologic therapy or electrical cardioversion.
Persistent AF may be either the first presentation of AF or the result of recurrent episodes of paroxysmal AF. Patients with persistent AF also include those with longstanding AF in whom cardioversion has not been indicated or attempted, often leading to permanent AF.
Patients can also have AF as an arrhythmia secondary to cardiac disease that affects the atria (eg, congestive heart failure, hypertensive heart disease, rheumatic heart disease, coronary artery disease). These patients tend to be older, and AF is more likely to be persistent.
Persistent AF with an uncontrolled, rapid ventricular heart rate response can cause a dilated cardiomyopathy and can lead to electrical remodeling in the atria (atrial cardiomyopathy). Therapy, such as drugs or atrioventricular nodal ablation and permanent pacemaker implantation, to control the ventricular rate can improve left ventricular function and improve quality-of-life scores.
Permanent AFPermanent AF is recognized as the accepted rhythm, and the main treatment goals are rate control and anticoagulation. While it is possible to reverse the progression from paroxysmal to persistent and to permanent, this task can be challenging.
Lone atrial fibrillationIn addition to the above schema, the term "lone atrial fibrillation" has been used to identify AF in younger patients without structural heart disease, who are at a lower risk for thromboembolism. The definition of lone AF remains controversial, but it generally refers to paroxysmal, persistent, or permanent AF in younger patients ([9]
PreviousNextPathophysiologyAtrial fibrillation (AF) shares strong associations with other cardiovascular diseases, such as heart failure, coronary artery disease (CAD), valvular heart disease, diabetes mellitus, and hypertension.[10] These factors have been termed upstream risk factors, but the relationship between comorbid cardiovascular disease and AF is incompletely understood and more complex than this terminology implies. The exact mechanisms by which cardiovascular risk factors predispose to AF are not understood fully but are under intense investigation. Catecholamine excess, hemodynamic stress, atrial ischemia, atrial inflammation, metabolic stress, and neurohumoral cascade activation are all purported to promote AF.
Because diabetes mellitus and obesity are increasing in prevalence and are associated with an elevated risk of AF, Fontes et al examined whether insulin resistance is an intermediate step for the development of AF. In a community-based cohort that included 279 patients who developed AF within 10 years of follow-up, no significant association was observed between insulin resistance and incident AF.[11]
Although the precise mechanisms that cause atrial fibrillation are incompletely understood, AF appears to require both an initiating event and a permissive atrial substrate. Significant recent discoveries have highlighted the importance of focal pulmonary vein triggers, but alternative and nonmutually exclusive mechanisms have also been evaluated. These mechanisms include multiple wavelets, mother waves, fixed or moving rotors, and macro-reentrant circuits. In a given patient, multiple mechanisms may coexist at any given time. The automatic focus theory and the multiple wavelet hypothesis appear to have the best supporting data.
Automatic focusA focal origin of AF is supported by several experimental models showing that AF persists only in isolated regions of atrial myocardium. This theory has garnered considerable attention, as studies have demonstrated that a focal source of AF can be identified in humans and that isolation of this source can eliminate AF.
The pulmonary veins appear to be the most frequent source of these automatic foci, but other foci have been demonstrated in several areas throughout the atria. Cardiac muscle in the pulmonary veins appears to have active electrical properties that are similar, but not identical, to those of atrial myocytes. Heterogeneity of electrical conduction around the pulmonary veins is theorized to promote reentry and sustained AF. Thus, pulmonary vein automatic triggers may provide the initiating event, and heterogeneity of conduction may provide the sustaining conditions in many patients with AF.
Multiple waveletThe multiple wavelet hypothesis proposes that fractionation of wave fronts propagating through the atria results in self-perpetuating "daughter wavelets." In this model, the number of wavelets is determined by the refractory period, conduction velocity, and mass of atrial tissue. Increased atrial mass, shortened atrial refractory period, and delayed intra-atrial conduction increase the number of wavelets and promote sustained AF. This model is supported by data from patients with paroxysmal AF demonstrating that widespread distribution of abnormal atrial electrograms predicts progression to persistent AF.[12] Intra-atrial conduction prolongation has also been shown to predict recurrence of AF.[13] Together, these data highlight the importance of atrial structural and electrical remodeling in the maintenance of AF—hence the phrase "atrial fibrillation begets atrial fibrillation."
PreviousNextEtiologyAtrial fibrillation (AF) is strongly associated with the following risk factors:
Hemodynamic stressAtrial ischemiaInflammationNoncardiovascular respiratory causesAlcohol and drug useEndocrine disordersNeurologic disordersGenetic factorsAdvancing ageHemodynamic stressIncreased intra-atrial pressure results in atrial electrical and structural remodeling and predisposes to AF. The most common causes of increased atrial pressure are mitral or tricuspid valve disease and left ventricular dysfunction. Systemic or pulmonary hypertension also commonly predisposes to atrial pressure overload, and intracardiac tumors or thrombi are rare causes.
Atrial ischemiaCoronary artery disease infrequently leads directly to atrial ischemia and AF. More commonly, severe ventricular ischemia leads to increased intra-atrial pressure and AF.
InflammationMyocarditis and pericarditis may be idiopathic or may occur in association with collagen vascular diseases; viral or bacterial infections; or cardiac, esophageal, or thoracic surgery.
Noncardiovascular respiratory causesPulmonary embolism, pneumonia, lung cancer, and hypothermia have been associated with AF.
Drug and alcohol useStimulants, alcohol, and cocaine can trigger AF. Acute or chronic alcohol use (ie, holiday or Saturday night heart, also known as alcohol-related cardiomyopathy) and illicit drug use (ie, stimulants, methamphetamines, cocaine) have been specifically found to be related to AF.
Endocrine disordersHyperthyroidism, diabetes, and pheochromocytoma have been associated with AF.
Neurologic disordersIntracranial processes such as subarachnoid hemorrhage or stroke can precipitate AF.
Familial AFA history of parental AF appears to confer increased likelihood of AF (and occasional family pedigrees of AF are associated with defined ion channel abnormalities, especially sodium channels).[14] One cohort study suggests that familial AF is associated with an increased risk of AF. This increase was not lessened by adjustment for genetic variants and other AF risk factors.[15]
Advancing ageAF is strongly age-dependent, affecting 4% of individuals older than 60 years and 8% of persons older than 80 years.
PreviousNextEpidemiologyAtrial fibrillation affects more than 2.2 million persons in the United States. AF is strongly age-dependent, affecting 4% of individuals older than 60 years and 8% of persons older than 80 years. Approximately 25% of individuals aged 40 years and older will develop AF during their lifetime.[16]
The prevalence of AF is 0.1% in persons younger than 55 years, 3.8% in persons 60 years or older, and 10% in persons 80 years or older. With the projected increase in the elderly population in the United States, the prevalence of AF is expected to more than double by the year 2050. AF is uncommon in childhood except after cardiac surgery.[17]
The incidence of AF is significantly higher in men than in women in all age groups. AF appears to be more common in whites than in blacks, with blacks have less than half the age-adjusted risk of developing AF.
In 10-15% of cases of AF, the disease occurs in the absence of comorbidities (lone atrial fibrillation). However, AF is often associated with other cardiovascular diseases, including hypertension; heart failure; diabetes-related heart disease; ischemic heart disease; and valvular, dilated, hypertrophic, restrictive, and congenital cardiomyopathies.[16] The Atherosclerosis Risk in Communities (ARIC) Study suggests reduced kidney function and presence of albuminuria are strongly associated with AF.[18]
The rate of ischemic stroke in patients with nonrheumatic AF averages 5% a year, which is somewhere between 2 and 7 times the rate of stroke in patients without AF. The risk of stroke is not due solely to AF; it increases substantially in the presence of other cardiovascular diseases.[19] The prevalence of stroke in patients younger than 60 years is less than 0.5%; however, in those older than 70 years, the prevalence doubles with each decade.[20] The attributable risk of stroke from AF is estimated to be 1.5% for those aged 50-59 years, and it approaches 30% for those aged 80-89 years. Women are at a higher risk of stroke due to AF than men and some have suggested this may be due to undertreatment with warfarin. However, one study of patients 65 years or older with recently diagnosed AF found warfarin use played no part in the increased risk of stroke among female patients.[21]
PreviousNextPrognosisAF is associated with a 1.5- to 1.9-fold higher risk of death, which is in part due to the strong association between AF and thromboembolic events, according to data from the Framingham heart study.[22]
Medical therapies aimed at rhythm control offered no survival advantage over rate control and anticoagulation, according to the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial. The study addressed whether rate control and anticoagulation are sufficient goals for asymptomatic, elderly patients.[23]
Atrial fibrillation (AF) is associated with increased morbidity and mortality, in part due to the risk of thromboembolic disease, particularly stroke, in AF and in part due to its associated risk factors. Studies have shown that individuals in sinus rhythm live longer than individuals with AF. Disruption of normal atrial electromechanical function in AF leads to blood stasis. This, in turn, can lead to development of thrombus, most commonly in the left atrial appendage. Dislodgement or fragmentation of a clot can then lead to embolic phenomena, including stroke.
Development of AF predicts heart failure and is associated with a worse New York Heart Association Heart Failure classification. AF may also worsen heart failure in individuals who are dependent on the atrial component of the cardiac output. Those with hypertensive heart disease and those with valvular heart disease are particularly at high risk for developing heart failure when AF occurs. In addition, AF may cause tachycardia-mediated cardiomyopathy if adequate rate control is not established.
The risk of stroke from AF that lasts longer than 24 hours is a major concern and is usually addressed by prescribing a blood thinner (Coumadin or dabigatran). Prognostic score systems, such as CHADS2, appear to underestimate the risk of embolic stroke in patients older than 75 years; thus, some studies recommend treating all patients older than 75 years unless a compelling contraindication is noted.[24] The CHADS2 score predicts ischemic stroke not only for patients with a history of atrial fibrillation but also for patients without atrial fibrillation who have a history of coronary heart disease.[25] In the latter group, net benefit of prophylactic anticoagulation has yet to be established.
An analysis of the AFNET (Central Registry of the German Competence NETwork on Atrial Fibrillation) registry of 8847 patients with nonvalvular atrial fibrillation indicated that the CHA2 DS2 -VASc score is more sensitive than the CHADS2 score for risk stratification of thromboembolic events (ischemic stroke, transient ischemic attack [TIA], systemic embolism), particularly in patients at low or intermediate risk for stroke (CHADS2 score of 0 or 1)—who therefore do not require oral anticoagulation.[26, 27]
During a mean follow-up of 5 years, the investigators found 36.5% (144 of 395) of strokes or other thromboembolic events occurred in patients given a CHADS2 score of 0 or 1, groups in which there is no definitive recommendation for oral anticoagulation.[26, 27] However, CHA2 DS2 -VASc scoring—which adds age 65-74 years, vascular disease, and female sex as stroke risk factors to the CHADS2 score[27] —placed 30.3% of those classified as CHADS2 0 or 1 into CHA2 DS2 -VASc 1 or 2 and higher, groups in which oral anticoagulation is recommended.[26]
A post-hoc analysis of the ONTARGET and TREND studies, which evaluated the efficacy of treatment with ramipril plus telmisartan or telmisartan alone in reducing cardiovascular disease, used the Mini–Mental State Examination (MMSE) to measure the cognitive function of participants at baseline and after two and five years. Results show that AF is associated with an increased risk of cognitive decline, new dementia, loss of independence in performing activities of daily living and admission to long-term care facilities.[28]
Atrial fibrillation in association with acute myocardial infarctionAF is a common finding in patients presenting with an acute myocardial infarction. A meta-analysis pooled data from 43 studies and more than 278,800 patients.[29] The study found that AF in the setting of acute myocardial infarction was associated with 40% increase in mortality compared to patients in sinus rhythm with acute myocardial infarction. The causes of death were unclear, but may be related to triple anticoagulation therapy with aspirin, clopidogrel, and warfarin, or may be related to hemodynamic consequences associated with the loss of atrial contraction. Whether AF is a complication of myocardial infarction or a marker for myocardial infarction severity is unclear.
PreviousNextPatient EducationA study by van Diepen et al suggests that patients with heart failure or atrial fibrillation have a significantly higher risk of noncardiac postoperative mortality than patients with coronary artery disease; thus, patients and physicians should consider this risk, even if a minor procedure is planned.[30]
For excellent patient education resources, visit eMedicineHealth's Heart Center and Stroke Center. Also, see eMedicineHealth's patient education articles Atrial Fibrillation, Heart Rhythm Disorders, Stroke, and Supraventricular Tachycardia.
PreviousProceed to Clinical Presentation  Contributor Information and DisclosuresAuthorLawrence Rosenthal, MD, PhD, FACC, FHRS Associate Professor of Medicine, Director, Section of Cardiac Pacing and Electrophysiology, Director of EP Fellowship Program, Division of Cardiovascular Disease, University of Massachusetts Memorial Medical Center
Lawrence Rosenthal, MD, PhD, FACC, FHRS is a member of the following medical societies: American College of Cardiology, American Heart Association, and Massachusetts Medical Society
Disclosure: Nothing to disclose.
David D McManus, MD, ScM, FACC, FHRS, Director, Atrial Fibrillation Program, Assistant Professor of Medicine and Quantitative Health Sciences, University of Massachusetts Medical School
Disclosure: Nothing to disclose.
Jeffrey N Rottman, MD Professor of Medicine and Pharmacology, Vanderbilt University School of Medicine; Chief, Department of Cardiology, Nashville Veterans Affairs Medical Center
Jeffrey N Rottman, MD is a member of the following medical societies: American Heart Association and North American Society of Pacing and Electrophysiology
Disclosure: Nothing to disclose.
Pierre Borczuk, MD Assistant Professor of Medicine, Harvard Medical School; Associate in Emergency Medicine, Massachusetts General Hospital
Pierre Borczuk, MD is a member of the following medical societies: American College of Emergency Physicians
Disclosure: Nothing to disclose.
David FM Brown, MD Associate Professor, Division of Emergency Medicine, Harvard Medical School; Vice Chair, Department of Emergency Medicine, Massachusetts General Hospital
David FM Brown, MD is a member of the following medical societies: American College of Emergency Physicians and Society for Academic Emergency Medicine
Disclosure: Nothing to disclose.
Abraham G Kocheril, MD, FACC, FACP, FHRS Professor of Medicine, University of Illinois College of Medicine
Abraham G Kocheril, MD, FACC, FACP, FHRS is a member of the following medical societies: American College of Cardiology, American College of Physicians, American Heart Association, American Medical Association, Cardiac Electrophysiology Society, Central Society for Clinical Research, Heart Failure Society of America, and Illinois State Medical Society
Disclosure: Nothing to disclose.
William Lober, MD, MS Associate Professor, Health Informatics and Global Health, Schools of Medicine, Nursing, and Public Health, University of Washington
Disclosure: Nothing to disclose.
Brian Olshansky, MD Professor of Medicine, Department of Internal Medicine, University of Iowa College of Medicine
Brian Olshansky, MD is a member of the following medical societies: American College of Cardiology, American Heart Association, Cardiac Electrophysiology Society, and Heart Rhythm Society
Disclosure: Guidant/Boston Scientific Honoraria Speaking and teaching; Medtronic Honoraria Speaking and teaching; Guidant/Boston Scientific Consulting fee Consulting
Gary Setnik, MD Chair, Department of Emergency Medicine, Mount Auburn Hospital; Assistant Professor, Division of Emergency Medicine, Harvard Medical School
Gary Setnik, MD is a member of the following medical societies: American College of Emergency Physicians, National Association of EMS Physicians, and Society for Academic Emergency Medicine
Disclosure: SironaHealth Salary Management position; South Middlesex EMS Consortium Salary Management position; ProceduresConsult.com Royalty Other
Ali A Sovari, MD, FACP Clinical and Research Fellow in Cardiovascular Medicine, Section of Cardiology, University of Illinois College of Medicine; Staff Physician and Hospitalist, St John Regional Medical Center, Cogent Healthcare, Inc
Ali A Sovari, MD, FACP is a member of the following medical societies: American College of Cardiology, American College of Physicians, American Heart Association, American Medical Association, American Physiological Society, and Heart Rhythm Society
Disclosure: Nothing to disclose.
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Salary Employment
ReferencesStiles S. Major Bleeds Less Daunting With Dabigatran Than Warfarin in Meta-Analysis. Medscape [serial online]. Oct 4 2013;Accessed Oct 15 2013. Available at http://www.medscape.com/viewarticle/812092.
Majeed A, Hwang HG, Connolly SJ, Eikelboom JW, Ezekowitz MD, Wallentin L, et al. Management and outcomes of major bleeding during treatment with dabigatran or warfarin. Circulation. Sep 30 2013;[Medline].
Zimetbaum P, Reynolds MR, Ho KK, Gaziano T, McDonald MJ, McClennen S, et al. Impact of a practice guideline for patients with atrial fibrillation on medical resource utilization and costs. Am J Cardiol. Sep 15 2003;92(6):677-81. [Medline].
Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. Aug 15 2006;114(7):e257-354. [Medline]. [Full Text].
[Guideline] Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. Jan 4 2011;123(1):104-23. [Medline].
[Guideline] Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS Focused Update on the Management of Patients With Atrial Fibrillation (Update on Dabigatran): A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. Feb 14 2011;[Medline].
[Best Evidence] Singh BN, Singh SN, Reda DJ, Tang XC, Lopez B, Harris CL, et al. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. May 5 2005;352(18):1861-72. [Medline].
Fuster V, Rydén LE, Asinger RW, et al. ACC/AHA/ESC Guidelines for the Management of Patients With Atrial Fibrillation: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation) Developed in Collaboration With the North American Society of Pacing and Electrophysiology. Circulation. Oct 23 2001;104(17):2118-50. [Full Text].
Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J Am Coll Cardiol. Aug 15 2006;48(4):854-906. [Medline].
Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. Oct 16 1998;82(8A):2N-9N. [Medline].
Fontes JD, Lyass A, Massaro JM, et al. Insulin Resistance and Atrial Fibrillation (from the Framingham Heart Study). Am J Cardiol. Jan 1 2012;109(1):87-90. [Medline]. [Full Text].
Nakao K, Seto S, Ueyama C, Matsuo K, Komiya N, Isomoto S, et al. Extended distribution of prolonged and fractionated right atrial electrograms predicts development of chronic atrial fibrillation in patients with idiopathic paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. Oct 2002;13(10):996-1002. [Medline].
Akyürek O, Sayin T, Dinçer I, Karaoguz R, Güldal M, Oral D. Lengthening of intraatrial conduction time in atrial fibrillation and its relation with early recurrence of atrial fibrillation. Jpn Heart J. Sep 2001;42(5):575-84. [Medline].
Fox CS, Parise H, D'Agostino RB Sr, Lloyd-Jones DM, Vasan RS, Wang TJ, et al. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. Jun 16 2004;291(23):2851-5. [Medline].
Lubitz SA, Yin X, Fontes JD, Magnani JW, Rienstra M, Pai M, et al. Association between familial atrial fibrillation and risk of new-onset atrial fibrillation. JAMA. Nov 24 2010;304(20):2263-9. [Medline].
Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. Aug 31 2004;110(9):1042-6. [Medline].
Abdel Latif A, Messinger-Rapport BJ. Should nursing home residents with atrial fibrillation be anticoagulated?. Cleve Clin J Med. Jan 2004;71(1):40-4. [Medline].
Alonso A, Lopez FL, Matsushita K, et al. Chronic Kidney Disease Is Associated With the Incidence of Atrial Fibrillation: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. Jun 28 2011;123(25):2946-53. [Medline].
Stöllberger C, Chnupa P, Abzieher C, Länger T, Finsterer J, Klem I, et al. Mortality and rate of stroke or embolism in atrial fibrillation during long-term follow-up in the embolism in left atrial thrombi (ELAT) study. Clin Cardiol. Jan 2004;27(1):40-6. [Medline].
Rathore SS, Berger AK, Weinfurt KP, Schulman KA, Oetgen WJ, Gersh BJ, et al. Acute myocardial infarction complicated by atrial fibrillation in the elderly: prevalence and outcomes. Circulation. Mar 7 2000;101(9):969-74. [Medline].
Avgil Tsadok M, Jackevicius CA, Rahme E, Humphries KH, Behlouli H, Pilote L. Sex differences in stroke risk among older patients with recently diagnosed atrial fibrillation. JAMA. May 9 2012;307(18):1952-8. [Medline].
Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. Aug 1991;22(8):983-8. [Medline].
Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. Dec 5 2002;347(23):1825-33. [Medline].
Hobbs FD, Roalfe AK, Lip GY, et al. Performance of stroke risk scores in older people with atrial fibrillation not taking warfarin: comparative cohort study from BAFTA trial. BMJ. Jun 23 2011;342:d3653. [Medline].
Welles CC, Whooley MA, Na B, et al. The CHADS(2) score predicts ischemic stroke in the absence of atrial fibrillation among subjects with coronary heart disease: Data from the Heart and Soul Study. Am Heart J. Sep 2011;162(3):555-61. [Medline].
Gerth A, Nabauer M, Oeff M, et al. Stroke events in patients with CHADS2 scores 0 and 1 in a contemporary population of patients with atrial fibrillation: results from the German AFNET registry [abstract 4381]. Presented at: European Society of Cardiology (ESC) Congress 2013; September 3, 2013; Amsterdam, The Netherlands. Eur Heart J. 2013;34 (suppl):808. [Full Text].
Hughes S. CHA2DS2-VASc score best for stroke risk assessment in AF. Medscape Medical News [serial online]. September 19, 2013;Accessed September 29, 2013. Available at http://www.medscape.com/viewarticle/811332.
Marzona I, O'Donnell M, Teo K, Gao P, Anderson C, Bosch J, et al. Increased risk of cognitive and functional decline in patients with atrial fibrillation: results of the ONTARGET and TRANSCEND studies. CMAJ. Feb 27 2012;[Medline].
Jabre P, Roger VL, Murad MH, et al. Mortality associated with atrial fibrillation in patients with myocardial infarction: a systematic review and meta-analysis. Circulation. Apr 19 2011;123(15):1587-93. [Medline].
van Diepen S, Bakal JA, McAlister FA, Ezekowitz JA. Mortality and readmission of patients with heart failure, atrial fibrillation, or coronary artery disease undergoing noncardiac surgery: an analysis of 38 047 patients. Circulation. Jul 19 2011;124(3):289-96. [Medline].
Michael JA, Stiell IG, Agarwal S, Mandavia DP. Cardioversion of paroxysmal atrial fibrillation in the emergency department. Ann Emerg Med. Apr 1999;33(4):379-87. [Medline].
Page RL, Wilkinson WE, Clair WK, McCarthy EA, Pritchett EL. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation. Jan 1994;89(1):224-7. [Medline].
van den Bos EJ, Constantinescu AA, van Domburg RT, Akin S, Jordaens LJ, Kofflard MJ. Minor elevations in troponin I are associated with mortality and adverse cardiac events in patients with atrial fibrillation. Eur Heart J. Mar 2011;32(5):611-7. [Medline].
Klein AL, Grimm RA, Murray RD, Apperson-Hansen C, Asinger RW, Black IW, et al. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med. May 10 2001;344(19):1411-20. [Medline].
Marrouche N. Delayed Enhancement - MRI determinant of successful Catheter Ablation of Atrial Fibrillation (DECAAF): analysis of post ablation scar and outcome. Presented at: The European Society of Cardiology (ESC) Congress 2013; September 1, 2013; Amsterdam, The Netherlands. [Full Text].
O'Riordan M. DECAAF: Targeting MRI-identified fibrosis during ablation improves outcomes. Heartwire [serial online]. September 1, 2013;Accessed September 17, 2013. Available at http://www.medscape.com/viewarticle/810308.
Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, et al. Thirty-Day Mortality After Ischemic Stroke and Intracranial Hemorrhage in Patients With Atrial Fibrillation On and Off Anticoagulants. Stroke. Apr 26 2012;[Medline].
Steinberg BA, Kim S, Piccini JP, Fonarow GC, Lopes RD, Thomas L, et al. Use and Associated Risks of Concomitant Aspirin Therapy with Oral Anticoagulation in Patients with Atrial Fibrillation: Insights from the ORBIT-AF Registry. Circulation. Jul 16 2013;[Medline].
van Walraven C, Hart RG, Wells GA, Petersen P, Koudstaal PJ, Gullov AL. A clinical prediction rule to identify patients with atrial fibrillation and a low risk for stroke while taking aspirin. Arch Intern Med. Apr 28 2003;163(8):936-43. [Medline].
Olesen JB, Lip GY, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. Jan 31 2011;342:d124. [Medline]. [Full Text].
Hagens VE, Ranchor AV, Van Sonderen E, Bosker HA, Kamp O, Tijssen JG, et al. Effect of rate or rhythm control on quality of life in persistent atrial fibrillation. Results from the Rate Control Versus Electrical Cardioversion (RACE) Study. J Am Coll Cardiol. Jan 21 2004;43(2):241-7. [Medline].
McNamara RL, Tamariz LJ, Segal JB, Bass EB. Management of atrial fibrillation: review of the evidence for the role of pharmacologic therapy, electrical cardioversion, and echocardiography. Ann Intern Med. Dec 16 2003;139(12):1018-33. [Medline].
Fang MC, Go AS, Chang Y, et al. A New Risk Scheme to Predict Warfarin-Associated Hemorrhage The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. Jul 19 2011;58(4):395-401. [Medline].
FDA. FDA approves Xarelto to prevent stroke in people with common type of abnormal heart rhythm. US Food and Drug Administration. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm278646.htm. Accessed November 4, 2011.
Bayer Schering Pharma AG. Xarelto: Summary of Product Characteristics. Available at http://www.xarelto.com/scripts/pages/en/information-on-xarelto/summary_of_product_characteristics/index.php. Accessed 2008.
Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. Sep 8 2011;365(10):883-91. [Medline].
Wallentin L, Yusuf S, Ezekowitz MD, Alings M, Flather M, Franzosi MG, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. Sep 18 2010;376(9745):975-83. [Medline].
O'Riordan M. Consistent benefit of apixaban, even in patients at highest risk of bleeding: ARISTOTLE. Medscape Medical News. Available at http://www.medscape.com/viewarticle/772080. Accessed October 15, 2012.
Lopes RD, Al-Khatib SM, Wallentin L, et al. Efficacy and safety of apixaban compared with warfarin according to patient risk of stroke and of bleeding in atrial fibrillation: a secondary analysis of a randomised controlled trial. Lancet. Oct 1 2012;[Medline].
Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. Sep 15 2011;365(11):981-92. [Medline]. [Full Text].
Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. Mar 3 2011;364(9):806-17. [Medline]. [Full Text].
O'Riordan M. FDA approves apixaban to prevent stroke in nonvalvular AF. Medscape Medical News [serial online]. Dec 28, 2012;Accessed January 9, 2013. Available at http://www.medscape.com/viewarticle/776846.
Lowes R. FDA Okays Kcentra to Reverse Anticoagulation, Stop Bleeding. Medscape Medical News. Available at http://www.medscape.com/viewarticle/803321. Accessed May 8, 2013.
O'Shea SI, Arcasoy MO, Samsa G, Cummings SE, Thames EH, Surwit RS, et al. Direct-to-patient expert system and home INR monitoring improves control of oral anticoagulation. J Thromb Thrombolysis. Aug 2008;26(1):14-21. [Medline].
[Best Evidence] van Walraven C, Hart RG, Connolly S, Austin PC, Mant J, Hobbs FD, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation: the atrial fibrillation investigators. Stroke. Apr 2009;40(4):1410-6. [Medline].
Gage BF, Yan Y, Milligan PE, Waterman AD, Culverhouse R, Rich MW, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. Mar 2006;151(3):713-9. [Medline].
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. Sep 17 2009;361(12):1139-51. [Medline].
Uchino K, Hernandez AV. Dabigatran Association With Higher Risk of Acute Coronary Events: Meta-analysis of Noninferiority Randomized Controlled Trials. Arch Intern Med. Jan 9 2012;[Medline].
Imazio M, Brucato A, Ferrazzi P, Rovere ME, Gandino A, Cemin R, et al. Colchicine Reduces Postoperative Atrial Fibrillation: Results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) Atrial Fibrillation Substudy. Circulation. Nov 22 2011;124(21):2290-2295. [Medline].
O'Riordan M. Colchicine postablation reduces early AF recurrences. Medscape Medical News. Available at http://www.medscape.com/viewarticle/772079. Accessed October 15, 2012.
Deftereos S, Giannopoulos G, Kossyvakis C, et al. Colchicine for prevention of early atrial fibrillation recurrence after pulmonary vein isolation: a randomized controlled study. J Am Coll Cardiol. Sep 22 2012;[Medline]. [Full Text].
[Best Evidence] Hansen ML, Sørensen R, Clausen MT, Fog-Petersen ML, Raunsø J, Gadsbøll N, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. Sep 13 2010;170(16):1433-41. [Medline].
Kowey PR, Reiffel JA, Ellenbogen KA, Naccarelli GV, Pratt CM. Efficacy and safety of prescription omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: a randomized controlled trial. JAMA. Dec 1 2010;304(21):2363-72. [Medline].
Liu T, Korantzopoulos P, Shehata M, Li G, Wang X, Kaul S. Prevention of atrial fibrillation with omega-3 fatty acids: a meta-analysis of randomised clinical trials. Heart. Jul 2011;97(13):1034-40. [Medline].
Shi y, Li D, Tardif JC, Nattel S. Enalapril effects on atrial remodeling and atrial fibrillation in experimental congestive heart failure. Cardiovasc Res. 2002;54:456-61.
Moreno I, Caballero R, Gonzalez T et al. Effects of irbesartan on cloned potassium channels involved in human cardiac repolarization. J Pharmacol Exp Ther. 2003;304:862-873.
Gerdts E, Wachtell K, Omvik, P et al. Left atrial size and risk of major cardiovascular events during antihypertensive treatment: Losartan Intervention for Endpoint Reduction in Hypertension trial. Hypertension. 2007;49:311-316.
Yusuf S, Healey JS, Pogue J, Chrolavicius S, Flather M, Hart RG, et al. Irbesartan in patients with atrial fibrillation. N Engl J Med. Mar 10 2011;364(10):928-38. [Medline].
[Best Evidence] Doyle JF, Ho KM. Benefits and risks of long-term amiodarone therapy for persistent atrial fibrillation: a meta-analysis. Mayo Clin Proc. Mar 2009;84(3):234-42. [Medline]. [Full Text].
Roy D, Talajic M, Dorian P, Connolly S, Eisenberg MJ, Green M, et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med. Mar 30 2000;342(13):913-20. [Medline].
FDA drug safety communication: Multaq (dronedarone) and increased risk of death and serious cardiovascular adverse events. July 21, 2011. U.S. Food and Drug Administration. Available at http://www.fda.gov/Drugs/DrugSafety/ucm264059.htm. Accessed July 26, 2011.
Connolly SJ, Camm AJ, Halperin JL, et al. Dronedarone in High-Risk Permanent Atrial Fibrillation. N Engl J Med. Nov 14 2011;[Medline].
Goodier R. Dofetilide cardioversion may increase proarrhythmia risk. Medscape Medical News [serial online]. June 3, 2013;Accessed June 11, 2013. Available at http://www.medscape.com/viewarticle/805227.
Brumberg G, Gera N, Pray C, Adelstein E, Barrington W, Bazaz R, et al. Frequency of Toxicity With Chemical Conversion of Atrial Fibrillation With Dofetilide. Am J Cardiol. May 22 2013;[Medline].
Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. Mar 21 1991;324(12):781-8. [Medline].
Hoyt H, Bhonsale A, Chilukuri K, Alhumaid F, Needleman M, Edwards D, et al. Complications arising from catheter ablation of atrial fibrillation: Temporal trends and predictors. Heart Rhythm. Dec 2011;8(12):1869-74. [Medline].
Boersma LV, Castella M, van Boven W, et al. Atrial Fibrillation Catheter Ablation Versus Surgical Ablation Treatment (FAST): A 2-Center Randomized Clinical Trial. Circulation. Jan 3 2012;125(1):23-30. [Medline].
[Best Evidence] Healey JS, Baranchuk A, Crystal E, Morillo CA, Garfinkle M, Yusuf S, et al. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis. J Am Coll Cardiol. Jun 7 2005;45(11):1832-9. [Medline].
Fauchier L, Pierre B, de Labriolle A, Grimard C, Zannad N, Babuty D. Antiarrhythmic effect of statin therapy and atrial fibrillation a meta-analysis of randomized controlled trials. J Am Coll Cardiol. Feb 26 2008;51(8):828-35. [Medline].
Vermes E, Tardif JC, Bourassa MG, Racine N, Levesque S, White M, et al. Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction: insight from the Studies Of Left Ventricular Dysfunction (SOLVD) trials. Circulation. Jun 17 2003;107(23):2926-31. [Medline].
Pedersen OD, Bagger H, Kober L, Torp-Pedersen C. Trandolapril reduces the incidence of atrial fibrillation after acute myocardial infarction in patients with left ventricular dysfunction. Circulation. Jul 27 1999;100(4):376-80. [Medline].
Alboni P, Botto GL, Baldi N, Luzi M, Russo V, Gianfranchi L, et al. Outpatient treatment of recent-onset atrial fibrillation with the "pill-in-the-pocket" approach. N Engl J Med. Dec 2 2004;351(23):2384-91. [Medline].
Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. [ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--excutive summary]. Rev Port Cardiol. Apr 2007;26(4):383-446. [Medline].
[Guideline] Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the Primary Prevention of Stroke. A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. Dec 6 2010;[Medline].
Bradley D, Creswell LL, Hogue CW Jr, Epstein AE, Prystowsky EN, Daoud EG. Pharmacologic prophylaxis: American College of Chest Physicians guidelines for the prevention and management of postoperative atrial fibrillation after cardiac surgery. Chest. Aug 2005;128(2 Suppl):39S-47S. [Medline].
Sezai A, Minami K, Nakai T, et al. Landiolol hydrochloride for prevention of atrial fibrillation after coronary artery bypass grafting: New evidence from the PASCAL trial. J Thorac Cardiovasc Surg. Jun 2011;141(6):1478-87. [Medline].
Anselme F, Saoudi N, Cribier A. Pacing in prevention of atrial fibrillation: the PIPAF studies. J Interv Card Electrophysiol. Jan 2000;4 Suppl 1:177-84. [Medline].
[Best Evidence] Roux JF, Zado E, Callans DJ, Garcia F, Lin D, Marchlinski FE, et al. Antiarrhythmics After Ablation of Atrial Fibrillation (5A Study). Circulation. Sep 22 2009;120(12):1036-40. [Medline].
Hussein AA, Wazni OM, Harb S, et al. Radiofrequency ablation of atrial fibrillation in patients with mechanical mitral valve prostheses safety, feasibility, electrophysiologic findings, and outcomes. J Am Coll Cardiol. Aug 2 2011;58(6):596-602. [Medline].
Onorati F, Mariscalco G, Rubino AS, Serraino F, Santini F, Musazzi A, et al. Impact of lesion sets on mid-term results of surgical ablation procedure for atrial fibrillation. J Am Coll Cardiol. Feb 22 2011;57(8):931-40. [Medline].
Haïssaguerre M, Shah DC, Jaïs P, Hocini M, Yamane T, Deisenhofer I, et al. Electrophysiological breakthroughs from the left atrium to the pulmonary veins. Circulation. Nov 14 2000;102(20):2463-5. [Medline].
Jaïs P, Shah DC, Haïssaguerre M, Hocini M, Garrigue S, Clémenty J. Atrial fibrillation: role of arrhythmogenic foci. J Interv Card Electrophysiol. Jan 2000;4 Suppl 1:29-37. [Medline].
Soga Y, Okabayashi H, Arai Y, et al. Up to 6-year follow-up after pulmonary vein isolation for persistent/permanent atrial fibrillation: Importance of sinus node function. J Thorac Cardiovasc Surg. Jun 2011;141(6):1455-60. [Medline].
O'Neill MD, Jaïs P, Hocini M, Sacher F, Klein GJ, Clémenty J, et al. Catheter ablation for atrial fibrillation. Circulation. Sep 25 2007;116(13):1515-23. [Medline].
Winkle RA, Mead RH, Engel G, Patrawala RA. Long-term results of atrial fibrillation ablation: The importance of all initial ablation failures undergoing a repeat ablation. Am Heart J. Jul 2011;162(1):193-200. [Medline].
Stiles S. Repeat Ablation Wins Out Over Antiarrhythmic Agents for Recurrent Paroxysmal. Medscape [serial online]. May 13 2013;Accessed May 23 2013. Available at http://www.medscape.com/viewarticle/804075.
Progression of Atrial Fibrillation After a Failed Initial Ablation Procedure in Patients With Paroxysmal Atrial Fibrillation: A Randomized Comparison of the Drug Therapy Versus Re-Ablation. ClinicalTrials.gov [serial online]. Accessed May 23 2013. Available at http://clinicaltrials.gov/ct2/show/NCT01709682.
Doshi RN, Daoud EG, Fellows C, Turk K, Duran A, Hamdan MH, et al. Left ventricular-based cardiac stimulation post AV nodal ablation evaluation (the PAVE study). J Cardiovasc Electrophysiol. Nov 2005;16(11):1160-5. [Medline].
Natale A, Zimerman L, Tomassoni G, Newby K, Leonelli F, Fanelli R, et al. AV node ablation and pacemaker implantation after withdrawal of effective rate-control medications for chronic atrial fibrillation: effect on quality of life and exercise performance. Pacing Clin Electrophysiol. Nov 1999;22(11):1634-9. [Medline].
Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. Aug 15 2009;374(9689):534-42. [Medline].
Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, et al. Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation. Nov 21 2000;102(21):2619-28. [Medline].
Schillig J, Kaatz S, Hudson M, et al. Clinical and safety impact of an inpatient Pharmacist-Directed anticoagulation service. J Hosp Med. Jul 2011;6(6):322-8. [Medline].
[Best Evidence] Connolly SJ, Pogue J, Hart RG, Hohnloser SH, Pfeffer M, Chrolavicius S, et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. May 14 2009;360(20):2066-78. [Medline].
Paré G, Mehta SR, Yusuf S, Anand SS, Connolly SJ, Hirsh J, et al. Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. N Engl J Med. Oct 28 2010;363(18):1704-14. [Medline].
Stiell IG, Dickinson G, Butterfield NN, Clement CM, Perry JJ, Vaillancourt C, et al. Vernakalant Hydrochloride: A NovelAtrial-selective Agent for the Cardioversionof Recent-onset Atrial Fibrillation in the Emergency Department. Acad Emerg Med. Nov 2, 2010;17(11):1175-1182.
Schmidt M, Christiansen CF, Mehnert F, Rothman KJ, Sorensen HT. Non-steroidal anti-inflammatory drug use and risk of atrial fibrillation or flutter: population based case-control study. BMJ. Jul 4 2011;343:d3450. [Medline].
Everett BM, Cook NR, Conen D, Chasman DI, Ridker PM, Albert CM. Novel genetic markers improve measures of atrial fibrillation risk prediction. Eur Heart J. Feb 26 2013;[Medline].
Nagy-Baló E, Tint D, Clemens M, Beke I, Kovács KR, Csiba L, et al. Transcranial Measurement of Cerebral Microembolic Signals during Pulmonary Vein Isolation: A Comparison of Two Ablation Techniques. Circ Arrhythm Electrophysiol. Apr 11 2013;[Medline].
Vassiliou VS, Flynn PD. Apixaban in atrial fibrillation: does predicted risk matter?. Lancet. Oct 1 2012;[Medline].
Â
 Ventricular rate varies from 130-168 beats per minute. Rhythm is irregularly irregular. P waves are not discernible. Classification scheme for patients with atrial fibrillation. Patient management for newly diagnosed atrial fibrillation. Subtherapeutic INR: INR Antiarrhythmic drug algorithm for the medical management of sinus rhythm in patients with atrial fibrillation. The image on the right is a reconstructed 3-dimensional image of the left atrium in a patient undergoing atrial fibrillation ablation. The figure on the left was created with a mapping catheter using Endocardial Solutions mapping technology. It represents the endocardial shell of the left atrium and is used as the template during left atrial ablation procedures. Table 1. Risk Factors for Stroke in Patients with Nonvalvular Atrial FibrillationTable 2. Adjusted Stroke Rate in Patients with Nonvalvular Atrial Fibrillation not Treated with AnticoagulationTable 3. Recommendations for Antithrombotic Therapy in Patients with Nonvalvular Atrial FibrillationTable 1. Risk Factors for Stroke in Patients with Nonvalvular Atrial FibrillationRisk FactorsRelative RiskPrior stroke or TIA2.5History of hypertension1.6Heart failure and/or reduced left ventricular function1.4Advanced age1.4Diabetes1.7Coronary artery disease1.5Table 2. Adjusted Stroke Rate in Patients with Nonvalvular Atrial Fibrillation not Treated with AnticoagulationCHADS2 ScoreAdjusted Stroke Rate (%/y)01.912.824.035.948.5512.5618.2Table 3. Recommendations for Antithrombotic Therapy in Patients with Nonvalvular Atrial FibrillationRisk CategoryRecommended TherapyNo risk factorsAspirin 81-325 mg dailyOne moderate-risk factorAspirin 81-325 mg daily or warfarin (INR 2-3)Any high-risk factor or more than 1 moderate-risk factorWarfarin (INR 2-3)
Ventricular rate varies from 130-168 beats per minute. Rhythm is irregularly irregular. P waves are not discernible. Classification scheme for patients with atrial fibrillation. Patient management for newly diagnosed atrial fibrillation. Subtherapeutic INR: INR Antiarrhythmic drug algorithm for the medical management of sinus rhythm in patients with atrial fibrillation. The image on the right is a reconstructed 3-dimensional image of the left atrium in a patient undergoing atrial fibrillation ablation. The figure on the left was created with a mapping catheter using Endocardial Solutions mapping technology. It represents the endocardial shell of the left atrium and is used as the template during left atrial ablation procedures. Table 1. Risk Factors for Stroke in Patients with Nonvalvular Atrial FibrillationTable 2. Adjusted Stroke Rate in Patients with Nonvalvular Atrial Fibrillation not Treated with AnticoagulationTable 3. Recommendations for Antithrombotic Therapy in Patients with Nonvalvular Atrial FibrillationTable 1. Risk Factors for Stroke in Patients with Nonvalvular Atrial FibrillationRisk FactorsRelative RiskPrior stroke or TIA2.5History of hypertension1.6Heart failure and/or reduced left ventricular function1.4Advanced age1.4Diabetes1.7Coronary artery disease1.5Table 2. Adjusted Stroke Rate in Patients with Nonvalvular Atrial Fibrillation not Treated with AnticoagulationCHADS2 ScoreAdjusted Stroke Rate (%/y)01.912.824.035.948.5512.5618.2Table 3. Recommendations for Antithrombotic Therapy in Patients with Nonvalvular Atrial FibrillationRisk CategoryRecommended TherapyNo risk factorsAspirin 81-325 mg dailyOne moderate-risk factorAspirin 81-325 mg daily or warfarin (INR 2-3)Any high-risk factor or more than 1 moderate-risk factorWarfarin (INR 2-3)
  View Table List Read more about Atrial Fibrillation on MedscapeRelated Reference Topics
 View Table List Read more about Atrial Fibrillation on MedscapeRelated Reference TopicsEmergent Management of Atrial Flutter
Flecainide Level
Implantable Loop Recorder
Related News and Articles
Marine n-3 Fatty Acids in Adipose Tissue and Development of Atrial Fibrillation
Caffeine Does Not Increase the Risk of Atrial Fibrillation
Dominant Frequency and Complex Fractionated Atrial Electrogram Ablation in Atrial Fibrillation
Medscape Reference © 2011 WebMD, LLC, Atrial Fibrillation
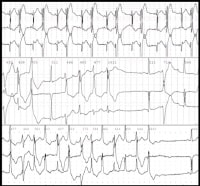 Bidirectional tachycardia in a patient with digitalis toxicity. NextBackground
Bidirectional tachycardia in a patient with digitalis toxicity. NextBackground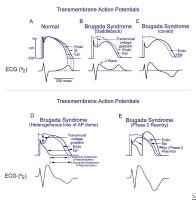 Schematics show the 3 types of action potentials in the right ventricle: endocardial (End), mid myocardial (M), and epicardial (Epi). A, Normal situation on V2 ECG generated by transmural voltage gradients during the depolarization and repolarization phases of the action potential. B-E, Different alterations of the epicardial action potential that produce the ECG changes observed in patients with Brugada syndrome. Adapted from Antzelevitch, 2005. NextBackground
Schematics show the 3 types of action potentials in the right ventricle: endocardial (End), mid myocardial (M), and epicardial (Epi). A, Normal situation on V2 ECG generated by transmural voltage gradients during the depolarization and repolarization phases of the action potential. B-E, Different alterations of the epicardial action potential that produce the ECG changes observed in patients with Brugada syndrome. Adapted from Antzelevitch, 2005. NextBackground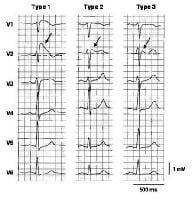 Three types of ST-segment elevation in Brugada syndrome, as shown in the precordial leads on ECG in the same patient at different times. Left panel shows a type 1 ECG pattern with pronounced elevation of the J point (arrow), a coved-type ST segment, and an inverted T wave in V1 and V2. The middle panel illustrates a type 2 pattern with a saddleback ST-segment elevated by >1 mm. The right panel shows a type 3 pattern in which the ST segment is elevated < 1 mm. According to a consensus report (Antzelevitch, 2005), the type 1 ECG pattern is diagnostic of Brugada syndrome. Modified from Wilde, 2002.
Three types of ST-segment elevation in Brugada syndrome, as shown in the precordial leads on ECG in the same patient at different times. Left panel shows a type 1 ECG pattern with pronounced elevation of the J point (arrow), a coved-type ST segment, and an inverted T wave in V1 and V2. The middle panel illustrates a type 2 pattern with a saddleback ST-segment elevated by >1 mm. The right panel shows a type 3 pattern in which the ST segment is elevated < 1 mm. According to a consensus report (Antzelevitch, 2005), the type 1 ECG pattern is diagnostic of Brugada syndrome. Modified from Wilde, 2002. 




