Acute pericarditis is an inflammation of the pericardium characterized by chest pain, pericardial friction rub, and serial ECG changes.
Signs and symptomsChest pain is the cardinal symptom of pericarditis, usually precordial or retrosternal with referral to the trapezius ridge, neck, left shoulder, or arm. Common associated signs and symptoms include low-grade intermittent fever, dyspnea/tachypnea (a frequent complaint and may be severe, with myocarditis, pericarditis, and cardiac tamponade), cough, and dysphagia. In tuberculous pericarditis, fever, night sweats, and weight loss are commonly noted (80%).
Specific causes of pericarditis include the following:
Idiopathic causesInfectious conditions, such as viral, bacterial, and tuberculous infectionsInflammatory disorders, such as RA, SLE, scleroderma, and rheumatic feverMetabolic disorders, such as renal failure, hypothyroidism, and hypercholesterolemiaCardiovascular disorders, such as acute MI, Dressler syndrome, and aortic dissectionMiscellaneous causes, such as iatrogenic, neoplasms, drugs, irradiation, cardiovascular procedures, and traumaSee Clinical Presentation for more detail.
DiagnosisInitial evaluation includes a clinical history and physical examination, ECG, echocardiography, chest radiography, and lab studies.
ECG can be diagnostic in acute pericarditis and typically shows ST elevation in all leads. The ratio of the amplitude of ST segment to the amplitude of the T wave in leads I, V4, V5, and V6 on electrocardiogram can be used to differentiate acute pericarditis (AP) from early repolarization (ER) and early repolarization of left ventricular hypertrophy (ERLVH), according to a recent study. When ST elevation was present in lead I, the ST/T ratio had the best predictive value for discriminating between AP, ER and ERLVH. The study involved 25 patients with AP, 27 with ER, and 28 with ERLVH.[1]
Echocardiography is particularly helpful if pericardial effusion is suspected on clinical or radiographic grounds, the illness lasts longer than 1 week, or myocarditis or purulent pericarditis is suspected.
A chest radiograph is only helpful for diagnosis in patients with effusions >250mL. Patients with small effusions (less than a few hundred milliliters) may present with a normal cardiac silhouette.
Lab tests may include CBC; serum electrolyte, blood urea nitrogen (BUN), and creatinine levels; erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels; and cardiac biomarker measurements, lactate dehydrogenase (LDH), and serum glutamic-oxaloacetic transaminase (SGOT; AST) levels.
See Workup for more detail.
ManagementTreatment for specific causes of pericarditis is directed according to the underlying cause. For patients with idiopathic or viral pericarditis, therapy is directed at symptom relief.
Pharmacologic treatment
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the mainstay of therapy. These agents have a similar efficacy, with relief of chest pain in about 85-90% of patients within days of treatment. A full-dose NSAID should be used, and treatment should last 7-14 days.
Colchicine, alone or in combination with an NSAID, can be considered for patients with recurrent or continued symptoms beyond 14 days.[2]
Corticosteroids should not be used for initial treatment of pericarditis unless it is indicated for the underlying disease, the patient’s condition has no response to NSAIDs or colchicine, or both agents are contraindicated.
Surgical treatment
Surgical procedures for pericarditis include pericardiectomy, pericardiocentesis, pericardial window placement, and pericardiotomy.
Pericardiectomy is the most effective surgical procedure for managing large effusions, because it has the lowest associated risk of recurrent effusions. This procedure is used for constrictive pericarditis, effusive pericarditis, or recurrent pericarditis with multiple attacks, steroid dependence, and/or intolerance to other medical management.
Patients with effusions larger than 250 mL, effusions in which size increases despite intensive dialysis for 10-14 days, or effusions with evidence of tamponade are candidates for pericardiocentesis.
Pericardial window placement is used for effusive pericarditis therapy. In critically ill patients, a balloon catheter may be used to create a pericardial window, in which only 9 cm2 or less of pericardium is resected.
Consider subxiphoid pericardiotomy for large effusions that do not resolve. This procedure may be performed under local anesthesia and has a lower risk of complications than pericardiectomy.
See Treatment and Medication for more detail.
Image library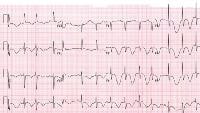 This 12-lead electrocardiogram is representative of pericarditis. NextBackground
This 12-lead electrocardiogram is representative of pericarditis. NextBackgroundAcute pericarditis is an inflammation of the pericardium characterized by chest pain, pericardial friction rub, and serial electrocardiographic (ECG) changes (see an example of such an ECG below). Pericarditis and cardiac tamponade involve the potential space surrounding the heart or pericardium; pericarditis is one cause of fluid accumulation in this potential space, and cardiac tamponade is the hemodynamic result of fluid accumulation.
 This 12-lead electrocardiogram is representative of pericarditis.
This 12-lead electrocardiogram is representative of pericarditis. For more information, see the the Medscape Reference articles Constrictive Pericarditis, Constrictive-Effusive Pericarditis, Pediatric Infective Pericarditis, and Imaging in Constrictive Pericarditis.
For patient education information, see the Cholesterol Center and Heart Center, as well as Pericarditis, Heart Attack, and Chest Pain.
PreviousNextAnatomyThe pericardium (pericardial complex) serves as a protective barrier from the spread of infection or inflammation from adjacent structures. It is composed of the parietal pericardium (an outer fibrous layer) and the visceral pericardium (an inner serous membrane made of a single layer of mesothelial cells). The fibrous pericardium is a flask-shaped, tough outer sac with attachments to the diaphragm, sternum, and costal cartilage. The visceral pericardium is thin, adjacent to the surface of the heart, and attached to the epicardial fat; it reflects back on itself to form the parietal pericardium.
The pericardium normally contains as much as 20-50 mL of an ultrafiltrate of plasma. Approximately 90-120 mL of additional pericardial fluid can accumulate in the pericardium without an increase in pressure. The capacity of the atria and ventricles to fill is mechanically compromised with further fluid accumulation, which can result in marked increases in pericardial pressure, eliciting reduced stroke volume, decreased cardiac output, and hypotension (cardiac tamponade physiology). The rapidity of fluid accumulation influences the hemodynamic effect. Drainage occurs via the thoracic duct and the right lymphatic duct into the right pleural space.
PreviousNextPathophysiologyPericardial physiology includes 3 main functions. First, through its mechanical function, the pericardium promotes cardiac efficiency by limiting acute dilation, maintaining ventricular compliance with preservation of the Starling curve, and distributing hydrostatic forces. The pericardium also creates a closed chamber with subatmospheric pressure that aids atrial filling and lowers transmural cardiac pressures. Second, through its membranous function, the pericardium shields the heart by reducing external friction and acting as a barrier against extension of infection and malignancy. Third, through its ligamentous function, the pericardium anatomically fixes the heart.
In most cases of acute pericarditis, the pericardium is acutely inflamed and has an infiltration of polymorphonuclear (PMN) leukocytes and pericardial vascularization. Often, the pericardium manifests a fibrinous reaction with exudates and adhesions. The pericardium may develop a serous or hemorrhagic effusion. A granulomatous pericarditis occurs with tuberculosis, fungal infections, rheumatoid arthritis (RA), and sarcoidosis.
Uremic pericarditis is thought to result from inflammation of the visceral and parietal layers of the pericardium by metabolic toxins that accumulate in the body owing to kidney failure. Other factors may be involved, however, because pericarditis also may occur in patients with chronic renal failure who are already receiving dialysis therapy.
The putative toxins suggested to precipitate uremic pericarditis when they accumulate are poorly characterized, but they may include urea, creatinine, methylguanidine, guanidinoacetate, parathyroid hormone, beta2-microglobulin, uric acid, and others. More than one toxin apparently may be involved, although considerable controversy surrounds this point.
The precise pathogenetic changes induced by these toxins when causing uremic pericarditis have not been elucidated, although a rough correlation with the degree and the duration of azotemia exists; the blood urea nitrogen (BUN) level is usually greater than 60 mg/dL (22 mmol/L). Uremic pericarditis may be associated with hemorrhagic or serous effusion, although considerable overlap exists. Hemorrhagic effusions are more common and result in part from uremia-induced platelet dysfunction.
Some authors distinguish between 2 types of pericarditis in patients with renal failure. One type is uremic pericarditis, which occurs in patients with uremia who have never received dialysis. The other type is dialysis-associated pericarditis, which occurs in patients who are already receiving dialysis. In the latter case, inadequate dialysis may usually be implicated, because aggressive dialysis often leads to resolution. Other causes of dialysis-associated pericarditis may include volume overload and bacterial or viral infections.
In an observational study that employed data from 88 maintenance hemodialysis patients, investigators found that intensive dialysis is the most effective treatment for dialysis-associated pericarditis in patients on dialysis who have diabetes and those who do not.[3] Following the intensification of hemodialysis, pericarditis improved in 85.1% of patients with diabetes and in 82.9% of those without diabetes. Among patients with diabetes, 85.1% survived without recurrence of pericarditis, 4.3% survived but did suffer recurrence, and 10.6% died, with similar outcomes recorded in the group without diabetes (87.8%, 4.9%, and 7.3%, respectively).[3]
PreviousNextEtiologyThis section will first briefly discuss acute pericarditis, chronic pericarditis, and cardiac tamponade; then, several specific entities that cause pericarditis will be briefly reviewed.
Acute pericarditisSerous pericarditis is usually caused by noninfectious inflammation such as occurs in rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). Fibrous adhesions rarely occur.
Fibrous and serofibrinous pericarditis represent the same basic process and are the most frequent type of pericarditis. Common causes include acute myocardial infarction (MI), postinfarction (including Dressler syndrome), uremia, radiation, RA, SLE, and trauma. Severe infections may also cause a fibrinous reaction, as does routine cardiac surgery.
Purulent or suppurative pericarditis due to causative organisms may arise from direct extension, hematogenous seeding, or lymphatic extension, or by direct introduction during cardiotomy. Immunosuppression facilitates this condition. Clinical features include fever, chills, and spiking temperatures. Constrictive pericarditis is a serious potential complication.
Hemorrhagic pericarditis involves blood mixed with a fibrinous or suppurative effusion, and it is most commonly caused by tuberculosis or direct neoplastic invasion. This condition can also occur in severe bacterial infections or in patients with a bleeding diathesis. Hemorrhagic pericarditis is common after cardiac surgery and may cause tamponade. The clinical significance is similar to suppurative pericarditis.
Until proven otherwise, caseation within the pericardial sac is tuberculous in origin. Untreated, caseous pericarditis is the most common antecedent to chronic constrictive pericarditis of a fibrocalcific nature.
Chronic pericarditisAdhesive mediastinopericarditis is a reaction that usually follows suppurative or caseous pericarditis, cardiac surgery, or irradiation. This condition is rarely caused by a simple fibrinous exudate. The pericardial potential space is obliterated, and adhesion of the external surface of the parietal layer to surrounding structures occurs. Clinically, systolic contraction of the ribcage and diaphragm and pulsus paradoxus may be observed. The increased workload may cause massive cardiac hypertrophy and dilatation, which can mimic an idiopathic cardiomyopathy.
Constrictive pericarditis is usually caused by suppurative, caseous, or hemorrhagic pericarditis. The heart may become encased in a 0.5-cm–thick to 1-cm–thick layer of scar or calcification (concretio cordis), resembling a plaster mold. Contrary to clinical findings in adhesive mediastinopericarditis, the heart cannot become hypertrophic or dilate because of insufficient space.
Imazio et al suggest that constrictive pericarditis is a rare complication of viral or idiopathic acute pericarditis ([4]
Cardiac tamponadeTamponade is more common in patients with malignant pericarditis. Effusions caused by tumors often progress to tamponade, eliciting bleeding in the pericardium. Blood accumulates more rapidly than a transudate or exudate and more commonly causes tamponade.
Identification of any pericardial fluid in the setting of penetrating injury to the thorax or upper abdomen requires aggressive resuscitation; penetrating cardiac injuries may occur, with hemopericardium as the most common feature. In acute massive hemopericardium, the time is insufficient for defibrination to occur. The hemopericardium organizes and may partially clot, resulting in a pericardial hematoma. The hematoma may appear echogenic instead of echo free.
Potential sources of iatrogenic cardiac perforation include central line placement, pacemaker insertion, cardiac catheterization, sternal bone marrow biopsies, and pericardiocentesis. The right atrium is the most common site of perforation from catheter placement. Perforation, as well as direct catheter infusion of fluids, can cause tamponade. In fact, a tamponade delay of hours to days has occurred secondary to catheter misplacement.
In one case report, tamponade was described as the first manifestation of dermatopolymyositis.[5]
Specific causes of pericarditis include the following and are briefly reviewed below:
Idiopathic causesInfectious conditions, such as viral, bacterial, and tuberculous infectionsInflammatory disorders, such as RA, SLE, scleroderma, and rheumatic feverMetabolic disorders, such as renal failure, hypothyroidism, and hypercholesterolemiaCardiovascular disorders, such as acute MI, Dressler syndrome, and aortic dissectionMiscellaneous causes, such as iatrogenic, neoplasms, drugs, irradiation, cardiovascular procedures, and traumaIdiopathic causesBetween 26% and 86% of cases of acute pericarditis are idiopathic in nature.[6] No clinical features distinguish idiopathic cases from viral pericarditis. It is likely that most idiopathic cases are undiagnosed viral infections. Seasonal peaks occur in spring and fall.
Chronic idiopathic pericarditis is defined as a pericardial effusion that persists more than 3 months without any apparent etiology. Pericardiocentesis alone results in resolution of large effusions; however, recurrence is common.
Viral infectionViral infection is the most common cause of acute pericarditis and accounts for 1-10% of cases. The disease is usually a short self-limited disease that lasts 1-3 weeks and can occur as seasonal epidemics, especially coxsackievirus B and influenza.
Causative viruses include coxsackievirus B,[7] echovirus, adenoviruses, influenza A and B viruses, enterovirus, mumps virus, Epstein-Barr virus, human immunodeficiency virus (HIV), herpes simplex virus (HSV) type 1, varicella-zoster virus (VZV), measles virus, parainfluenza virus (PIV) type 2, and respiratory syncytial virus (RSV), cytomegalovirus (CMV), and hepatitis viruses A, B, and C (HAV, HBV, HCV, respectively).
Patients may have associated myocarditis. Pericardial involvement is frequent in persons with HIV, but is usually an asymptomatic pericardial effusion of small volume. Individuals with advanced HIV infection develop pericardial involvement more frequently, with one study noting right atrial diastolic compression in 5% of cases involving advanced HIV infection.[8] Symptomatic pericarditis occurs in less than 1% of cases involving HIV, and its etiology can include the usual causes, opportunistic infection, Kaposi sarcoma, and HIV.
Bacterial infectionBacterial infections accounts for 1-8% of pericarditis cases and result from direct pulmonary extension, hematogenous spread, myocardial abscess or endocarditis, penetrating injury to chest wall from either trauma or surgery, or a subdiaphragmatic suppurative lesion. Purulent pericarditis may result from previous aseptic pericarditis, and a high percentage of patients develop constrictive pericarditis.
Organisms that have been isolated include gram-positive species such as Streptococcus pneumoniae and other Streptococcus species and Staphylococcus.[9] Isolated gram-negative species include Proteus, Escherichia coli, Pseudomonas, Klebsiella, Salmonella, Shigella, Neisseria meningitidis, and Haemophilus influenzae.
Less common organisms include Legionella, Nocardia, Actinobacillus, Rickettsia, Borrelia burgdorferi (Lyme borreliosis), Listeria, Leptospira, Chlamydophila psittaci, and Treponema pallidum (syphilis).
Anaerobes have also been isolated in 40% of patients in reviews of the pediatric population.
Previously, Pneumococcus was the predominant organism. However, in the antibiotic era, staphylococcal and gram-negative species have become more common. Most cases are now associated with thoracic surgery, renal disease, and immunosuppression.
Tuberculous infectionTuberculosis accounts for 4% of cases and should be considered in all instances of pericarditis without a rapid course, especially in high-risk groups, such as elderly patients in nursing homes and those with acquired immunodeficiency syndrome (AIDS).[10] Approximately 50% of affected patients develop constrictive pericarditis.
Fungal and parasitic infectionFungal organisms that may cause acute pericarditis include Histoplasma, Blastomyces, Coccidioides, Aspergillus, and Candida. Parasitic organisms include Entamoeba, Echinococcus, and Toxoplasma.
Rheumatoid arthritisPericarditis occurs predominantly in males with severely destructive and nodular RA. The pericardial involvement is usually clinically silent, with the diagnosis made in only 2% of adults and 6% of juveniles with RA. Rarely, pericarditis precedes the onset of RA. Autopsy studies show a pericarditis prevalence of 11-50%.
Systemic lupus erythematosus, scleroderma, sarcoidosisClinically evident pericarditis has been reported in 25% of patients with SLE and usually occurs in lupus flare-ups, but it may be the presenting manifestation. Autopsy series reveal pericardial involvement in 62% of lupus patients.
Pericarditis is recognized in 5-10% of patients with scleroderma, with a 70% autopsy prevalence. Pericardial effusions occur in 40% of patients with scleroderma and can be due to scleroderma, myocardial failure (restrictive cardiomyopathy), and renal failure. Restrictive cardiomyopathy and pericardial constriction can coexist. Usually, pulmonary hypertension, right heart failure, and systolic dysfunction occur.
Sarcoidosis may result in pericarditis, but this condition rarely causes cardiac tamponade or constrictive pericarditis
Rheumatic feverPericarditis in those with rheumatic fever occurs more commonly in lower socioeconomic groups and in children, often accompanying endocarditis and myocarditis, with a worse prognosis. Consider rheumatic fever as an etiology in any child with pericarditis. However, this disease is not a demonstrated cause of constrictive pericarditis.
In adults, pericarditis may not occur with myocardial or valvular involvement, and it is associated with a better prognosis. The pericarditis usually appears 7-10 days after the onset of fever and arthritis. Often, stage 1 electrocardiographic (ECG) findings are absent (see Electrocardiography).
Other inflammatory conditionsThe following conditions may also cause acute pericarditis:
Sjögren syndromeMixed connective-tissue diseaseReiter syndromeAnkylosing spondylitisInflammatory bowel diseaseWegener granulomatosisVasculitis (eg, giant cell arteritis, polyarteritis)PolymyositisBehçet syndromeWhipple diseaseFamilial Mediterranean feverSerum sicknessRenal failureRichard Bright described uremic pericarditis in 1836. Since that classic description, this common complication of chronic renal failure has evolved from an ominous event heralding the terminal stages of disease to an event that, with early management, is likely to have a good outcome. Furthermore, advances in dialysis technology with early and timely management of chronic renal failure have dramatically reduced the prevalence of uremic pericarditis. Uremic pericarditis has a prevalence of 6-10% in patients with acute or chronic renal failure, and it continues to be associated with significant morbidity and occasional mortality.
Renal failure accounts for approximately 12% of cases of pericarditis. In the predialysis era, pericarditis developed in 35-50% of patients with uremia who had chronic renal failure and less commonly in those with acute renal failure. Death often followed in several weeks. With dialysis, the pericarditis incidence rate is less than 10%; however, this condition occurs after the onset of dialysis in 8-12% of cases.
Asymptomatic pericardial effusions can occur in 36-62% of patients with uremia who require dialysis; these effusions are often small to moderate in size and can occur secondary to volume overload. Pericardial effusions can lead to significant hemodynamic complications during routine dialysis. Moreover, the presence of a large pericardial effusion that persists for longer than 10 days after intensive dialysis has a high likelihood of causing tamponade.
HypothyroidismHypothyroidism accounts for as many as 4% of pericarditis cases. In fact, myocardial involvement is common, and pericardial involvement usually occurs with severe hypothyroidism. Patients may develop large pericardial effusions, but they rarely develop tamponade.
Cholesterol pericarditisCholesterol pericarditis, also called gold-paint pericarditis, is a complication of a chronic pericardial effusion exacerbated by cholesterol crystals. It usually presents with large effusions that are not hemodynamically important, and development of constriction is rare. Granulomatous pericarditis has been implicated in some cases.
Myocardial infarctionAfter a transmural infarction, a fibrinous pericardial exudate appears within 24 hours, begins to organize at 4-8 days, and completes organization at 4 weeks.[11, 12] Pericardial pain occurs less frequently than the friction rub, which is often detected on the second or third day after an acute MI but may be heard within 24 hours and as late as 10 days.
Before thrombolytic therapy, infarct-associated pericarditis ranges from 7% to 23% of cases. At autopsy in one study, almost all patients were noted to have localized fibrinous pericarditis overlying the area of infarction. With thrombolytic therapy and direct infarct angioplasty, the incidence of post–MI-associated pericarditis has decreased to 5-8%.
Overall, pericardial involvement indicates a larger infarction, greater incidence of left ventricular dysfunction, and greater mortality. The pericarditis usually heals without consequence; effusions may occur, but they rarely lead to tamponade.
Dressler syndromeDressler syndrome is now considered rare. When pericarditis associated with Dressler syndrome does occur, it is usually observed 2-3 weeks after a myocardial infarction. Initially, the syndrome was described in as many as 4% of patients following and acute MI. Later studies suggested a much lower incidence. Dressler syndrome is rarely described with pulmonary embolism.
This syndrome may be a unique autoimmune-mediated phenomenon to myocardial antigens, or it may merely be an unrecognized post–MI pericarditis. Patients may develop pulmonary infiltrates and large pericardial effusions.
Because of the risk of hemorrhagic pericarditis, anticoagulant therapy should be stopped in patients with Dressler syndrome.
Aortic dissection and Takotsubo cardiomyopathyAortic dissection accounts for 1% of cases of acute pericarditis, especially for cases with hemorrhage into the pericardium.
Takotsubo cardiomyopathy is a transient cardiac syndrome that involves left ventricular apical akinesis and mimics acute coronary syndrome.
NeoplasmMalignancy account for 5-17% of pericarditis cases; in patients presenting with acute pericarditis or pericardial effusion, 4-7% have an unsuspected malignancy. Primary neoplasm of the heart and pericardium is rare; most cases of neoplasm-related pericarditis are a result of metastatic disease. Autopsy studies have noted that approximately 10% of patients with cancer develop cardiac involvement, and it is often clinically silent. The neoplastic cells reach the pericardium through the bloodstream, through the lymphatic system, or via local growth.
Neoplastic disease, particularly advanced disease, is the most frequent cause of tamponade in the hospital. Occasionally, the tumor encases the heart and causes constrictive pericarditis rather than tamponade.
Pericardial mesothelioma and angiosarcoma are lethal malignancies with aggressive local spread that respond poorly to treatment. Infants and children can present with a teratoma in the pericardial space. These can often be successfully removed.
Lung cancer, including adenocarcinoma and squamous and small cell carcinoma, accounts for approximately 33% of cases; breast cancer accounts for 25%; leukemia and lymphoma, including Hodgkin and non-Hodgkin, account for 15% of cases; and malignant melanoma represents another 5%. Almost all other malignancies, except primary brain, comprise the rest of the cases. Kaposi sarcoma has also become a more prominent cause of neoplastic disease with the AIDS epidemic.
DrugsSome medications, including penicillin and cromolyn sodium, induce pericarditis through a hypersensitivity reaction. The anthracycline antineoplastic agents, such as doxorubicin and cyclophosphamide, have direct cardiac toxicity and can cause acute pericarditis and myocarditis.
Pericarditis can also develop from a drug-induced lupus syndrome caused by medications including procainamide, hydralazine, methyldopa, isoniazid, mesalazine, and reserpine. Methysergide causes constrictive pericarditis through mediastinal fibrosis. Dantrolene, phenytoin, and minoxidil produce pericarditis through an unknown mechanism.
Smallpox vaccination infrequently leads to myocarditis. In a review of a large vaccination program in the US military, approximately 12 per 100,000 vaccinated troops developed myopericarditis within 14 days of vaccination.[13, 14] Whether this was due to a direct viral cytopathic effect or an immune-mediated phenomenon is unclear.
IrradiationPericardial disease is the most common cardiac toxicity from radiation therapy. Others are coronary artery disease, conduction disturbance, and myocardial and valvular disease.[15] A high incidence of such toxicity occurs with high doses, especially those greater than 4000 rad.
Radiation pericarditis can present as acute pericarditis, with or without effusion; chronic constrictive pericarditis; or effusive-constrictive pericarditis.
Invasive cardiac proceduresElectrophysiologic studies, radiofrequency ablation, pacemaker implantation, and percutaneous coronary intervention are among several invasive cardiac procedures that can cause pericarditis.
Postpericardiotomy syndrome is similar to Dressler syndrome, except that postpericardiotomy syndrome occurs after cardiac surgery. Several series note an incidence rate of 10-40%; approximately 1% of patients with postpericardiotomy syndrome develop tamponade.
Pericardial effusions can occur in the absence of typical features of postpericardiotomy syndrome. In one study, 56% developed pericardial effusions early after cardiac surgery, without correlation to pericarditis or tamponade. The effusions were more common after heavy postoperative bleeding.
TraumaApproximately 1% of cases of acute pericarditis are caused by trauma, such as penetrating and nonpenetrating cardiac trauma. Also consider esophageal rupture or perforation and pancreatitis.
PreviousNextEpidemiologyEpidemiologic data on the incidence of acute pericarditis are lacking, likely because this condition is frequently inapparent clinically, despite its presence in numerous disorders. Lorell noted a diagnosis of acute pericarditis in approximately 1 per 1000 hospital admissions.[16] In addition, acute pericarditis comprises 1% of emergency room visits in patients with ST-segment elevation.[17] In fact, the reported incidence of acute pericardial tamponade is approximately 2% of penetrating trauma; however, this condition is rarely seen in blunt chest trauma.
Uremic pericarditis may occur in 6-10% of patients with advanced renal failure before initiation of dialysis. When patients with large effusions are studied, uremia may account for up to 20% of cases in some series. The widespread availability of dialysis has reduced the incidence of uremic pericarditis.
Malignant disease is the most common cause of pericardial effusion with tamponade in developed countries; However, tuberculosis should be considered in endemic areas.
Acute pericarditis is more common in men than in women. However, although this condition is more common in adults than in children, adolescents are more commonly affected than young adults. Nonetheless, Merce et al found no difference in etiology, clinical course, and prognosis between elderly and younger patients with moderate and large pericardial effusions.[18]
PreviousNextPrognosisThe prognosis in individuals with pericarditis depends on the etiology of this condition, as well as the presence of a pericardial effusion and/or tamponade. Idiopathic and viral etiologies usually have a self-limited course, without any risk of evolution toward constrictive pericarditis.[19, 20] Most post–MI cases have a benign course; however, pericarditis is associated with larger infarcts, and therefore, overall long-term mortality may be increased.
Patients with scleroderma or children with rheumatic fever and pericarditis have a poor prognosis, and purulent, tuberculous, and neoplastic pericardial involvement have more complicated courses with worse outcomes. Purulent pericarditis is associated with a mortality rate nearing 100% for untreated persons and a mortality rate of 12-40% for treated patients. The mortality rate in tuberculous pericarditis approaches 50%.
Uremic pericarditis continues to be associated with significant morbidity and occasional mortality. Of patients with uremic pericarditis, 3-5% may develop hemorrhagic pericarditis.
For penetrating injuries, the prognosis depends heavily on the rapid identification of tamponade. Mortality may occur in 3-5% of cases resulting from cardiac tamponade or arrhythmias. Favorable factors include minor perforations, isolated right ventricular wounds, systolic blood pressure more than 50 mm Hg, and the presence of tamponade.
PreviousProceed to Clinical Presentation , Acute Pericarditis





0 comments:
Post a Comment