The 2 primary modalities for revascularization are coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI). This article briefly discusses the history, indications, applications, and current status of these revascularization procedures. Rapid advances are being made in both PCI and surgical revascularization, and such advances are likely to continue in the years to come.
Completely bioabsorbable stent struts are being developed.[1] Bioabsorbable magnesium stents are being evaluated.[2, 3] The advantages of biodegradable polymers include high drug-loading capacity, controlled long-term drug release, and full degradation of the polymer over a defined period, resulting in full release of the drug during a well-controlled time interval.[3]
Thromboresistant polymer coatings based on biologic substances such as fibrin, collagen, hyaluronic acid, and biologic oils are being tested. These polymers serve as drug delivery reservoirs for drugs that inhibit neointimal hyperplasia through suppression of platelet activation and the inflammatory response and through inhibition of smooth muscle cell migration and proliferation.
The effect of variable dose and release kinetics of drugs on neointimal hyperplasia is also being studied.[4] Multiple drugs may be delivered at timed intervals through newly designed stents.
Gene-eluting stents[5] are undergoing experimental and clinical trials; these will be usable either alone or in conjunction with other drug-eluting stents (DESs) and may further reduce in-stent restenosis (ISR). The ABSORB trial studied the safety of the bioabsorbable everolimus-eluting stent.[6] At 2 years, the stent was bioabsorbed, with vasomotion restored and restenosis prevented, and was clinically safe, suggesting freedom from late thrombosis.
The TRITON-TIMI 38 trial[7] showed that in patients with acute coronary syndromes with scheduled PCI, prasugrel therapy was associated with significantly reduced rates of ischemic events, including stent thrombosis, but with an increased risk of major bleeding, including fatal bleeding. Overall mortality did not differ significantly between treatment groups. Prasugrel has been approved by the US Food and Drug Administration (FDA). Other antithrombotics are in the pipeline.
Most acute coronary syndromes are caused by rupture of unstable plaques of mild-to-moderate stenosis ([8] Stenting of such vulnerable lesions might result in stabilization and prevention of plaque rupture. Likewise, rapid advances have been made in surgical techniques. In the future, PCI and CABG may come to be seen as complementary techniques for myocardial revascularization.
NextSurgical RevascularizationIn 1953, William Mustard performed the first direct surgical approach to the coronary circulation: a carotid-to-coronary bypass in a patient in Toronto. In 1962, the first surgical myocardial revascularization procedure, the patch graft technique, was performed to repair an obstruction of the left main trunk coronary artery.[9] Subsequently, saphenous vein graft (SVG) interposition became the dominant approach.
Shortly thereafter, coronary artery bypass grafting (CABG) became the most commonly performed surgical procedure in the United States. Subsequently, advantages of using the left internal mammary artery (LIMA) were demonstrated.[10] Better long-term patency rates and improved late survival were achieved by using the LIMA than by using SVGs.
Since 1986, the LIMA has been used in more than 90% of CABG procedures. Less frequently, the right internal mammary artery (RIMA) is used. However, a large study found that the RIMA is as good as the LIMA for CABG and better than either radial arteries or veins. Radial artery grafts have about the same 1-year patency rates as SVGs.[11]
Use of both the RIMA and the LIMA raises concerns about possible impaired sternotomy healing and infection, but it may be considered if the risk of not using both vessels outweighs the 2.5% risk of infection (eg, if the patient does not have good vein conduits and the LIMA is not sufficient for the number of bypass touchdown sites needed).[12] Most CABG operations involve placement of 1 or more SVG grafts in addition to use of the LIMA.
IndicationsIn 2004, the American College of Cardiology (ACC) and the American Heart Association (AHA) published guidelines for CABG, which divide indications into 3 classes.[13]
Class I indications are as follows:
Left main coronary artery disease (LMCAD) with 50% or greater narrowingAnatomically equivalent LMCAD with 70% or greater narrowing in both the proximal left anterior descending (LAD) coronary artery and the left circumflex artery 3-vessel coronary artery disease (CAD), particularly in the setting of an impaired left ventricular ejection fraction (LVEF)Class II indications are as follows:
Proximal LAD coronary artery stenosis (impaired LVEF becomes a class I indication)1-vessel or 2-vessel CAD that does not involve the proximal LAD coronary artery if a moderate area of viable myocardium is at riskClass III indications are as follows:
1-vessel or 2-vessel CAD that does not involve the proximal LAD coronary artery1-vessel or 2-vessel CAD that does not involve the proximal LAD coronary artery with only a small area of viable myocardiumThe expected benefit from surgery must be weighed against its morbidity and mortality. Improvements in operative techniques and technologies have allowed surgical treatment of more difficult cases.
Early studies comparing CABG with coronary intervention for multivessel disease demonstrated that the 2 strategies had similar mortalities but that there was an increased need for repeat procedures in the angioplasty and bare-metal stent (BMS) arms.[14, 15] Some studies have demonstrated improved mortality in diabetic subgroups that undergo surgical revascularization, though those findings have not been universal.
Before the widespread use of drug-eluting stents, the main advantages of CABG over percutaneous coronary intervention (PCI) included a lower rate of repeat procedures, greater success with chronically occluded coronary arteries, and protection of the entire vessel proximal to the distal anastomosis of a mammary graft. The primary disadvantage of CABG is the higher rate of upfront risks and complications.
Drug-eluting stents have improved PCI results, particularly with regard to repeat revascularization, and there is hope that a decrease in restenosis with drug-eluting stents (DESs) will reduce the need for repeat procedures in patients with multivessel disease that was treated with PCI. Large clinical trials comparing surgery and PCI with DESs (eg, SYNTAX, CARDIA, FREEDOM, and VA CARDS)[16, 17, 18] should provide more data on this critical issue.
Results from the SYNTAX trial showed that the rates of major adverse cardiac or cerebrovascular events at 12 months were significantly higher in the PCI group (17.8% vs 12.4% for CABG), in large part because of an increased rate of repeat revascularization (13.5% vs 5.9%).[16] At 12 months, the rates of death and myocardial infarction (MI) were comparable in the 2 groups; stroke was significantly more likely to occur with CABG (2.2% vs 0.6% with PCI).
Minimally invasive coronary artery bypass graftingAdvances in CABG have enabled surgeons to avoid having to perform a median sternotomy, thereby reducing pain and respiratory complications and preventing the large scar associated with this incision. Minimally invasive CABG includes surgical techniques that allow access to the heart through small thoracotomy incisions with endoscopic robotic surgery using a computer-enhanced telemanipulation system.[19, 20, 21]
Off-pump CABG (OPCABG) usually involves a medial sternotomy but avoids cardiopulmonary bypass (CPB), thus perhaps reducing bleeding and the frequency of renal failure. Minimally invasive direct CABG (MIDCABG) can be performed with or without CPB.
Robotic CABG
In robotic CABG, the surgeon, seated at a computer console, introduces instruments through small incisions in the chest and manipulates them with robotic arms. Early results of robotic coronary endoscopic surgery were successful on anterior vessels, and this success led to the development of complete multivessel endoscopic CABG.[19, 20, 21]
Minimally invasive direct CABG
In MIDCABG without CPB, the LIMA is harvested under direct visualization through a small left anterior thoracotomy incision or via an endoscopic approach through a small porthole incision. One end of a free segment of the inferior epigastric artery, the radial artery, or the saphenous vein is attached in an end-to-side fashion to the LIMA (which is anastomosed to the left anterior descending [LAD] artery), and the other end extends the bypass to other accessible coronary arteries (eg, diagonal or circumflex branches), in the form of a T or Y graft.[22]
Small thoracic incisions substantially reduce the morbidity related to median sternotomy and offer a quicker recovery, though wound complications occur in as many as 9% of patients.[23] Although other arteries can be accessed, the anterior MIDCABG approach is best suited for the anterior coronary vessels.
MIDCABG without CPB is primarily performed in the following circumstances:
For anterior lesions (lesions of the LAD in particular) when PCI is unsuitable for technical or other reasonsFor patients in whom traditional bypass cannot be performed safely because of major comorbidities associated with high surgical risk[24] For reoperation, when sternotomy or CPB is contraindicated because of the risk of jeopardizing bypass grafts, cardiac structures adherent to the sternum, previous sternal wound infection, mediastinal radiation therapy, or a calcified or diffusely atherosclerotic aorta[25, 26]MIDCABG to the LAD in conjunction with PCI of the right coronary artery or left circumflex artery is also performed.[27, 28] This procedure is done off the pump through a small anterior thoracotomy. Typically, MIDCABG offers access only to the LAD and diagonal coronary arteries. Hybrid MIDCABG is not widely used, because of limited exposure and the increased difficulty of harvesting the mammary artery. Furthermore, thoracotomy may cause more pain than sternotomy.
When MIDCABG is done through a limited anterior thoracotomy with CPB, antegrade and retrograde cardioplegia is delivered to produce optimal myocardial protection. The empty decompressed heart is still and bloodless. This approach offers enhanced myocardial protection, better access, and greater freedom to manipulate and expose the entire heart, which is necessary for multivessel CABG.
Off-pump CABG
OPCABG is performed via a conventional median sternotomy, which facilitates harvest of the internal mammary artery and access to all coronary arteries. Specialized cardiac retraction and myocardial stabilization platforms improve the accuracy and ease of distal anastomosis on the beating heart,[29, 30] facilitating the achievement of complete standard anastomoses. Temporary endovascular shunts are used to limit myocardial ischemia.
In the Surgical Management of Arterial Revascularization Therapies (SMART) trial, 197 patients were randomly assigned to either OPCABG or conventional CABG performed by a single surgeon; a mean of 3.2 grafts per patient were placed.[31] Rates of death, stroke, MI, recurrent angina, and revascularization at 30 days and 1 year were similar. The quality of life was comparable in the 2 groups, and the cost of care was modestly reduced with OPCABG.
The main advantage of OPCABG is that it prevents complications of artificial perfusion and CPB, including reductions in inflammatory response, postoperative infection, and atrial fibrillation.[32, 33]
The Society of Thoracic Surgeons database revealed that CABG without CPB (ie, beating-heart surgery) represented 10% of all CABG procedures in the United States from 1998 to 1999.[34] This number increased to approximately 25% of isolated CABG procedures in 2001.[35]
In a single-center randomized trial, 308 patients undergoing CABG were randomly assigned to OPCABG and CPB; no difference was found between groups with respect to the combined endpoint of death, MI, further revascularization (surgery or angioplasty), or stroke at 5-year follow-up.[36]
Port-access CABG
In port-access CABG, the grafting is performed through small incisions, but with conventional CPB. The connections to the bypass machine are made through the femoral vessels rather than through the open chest. This approach is time-consuming, technically difficult, and potentially risky for patients with lower-extremity arterial or venous insufficiency. Consequently, it has not been widely applied.
PreviousNextPercutaneous Coronary InterventionIn 1977, Andreas Grüntzig performed the first coronary angioplasty as a nonsurgical method for coronary artery revascularization.[37] In 2005, more than 600,000 percutaneous coronary intervention (PCI) procedures were performed in the United States. Originally, angioplasty was performed only in stable patients with a single discrete, noncalcified, proximal, concentric lesion in a single vessel; currently, such patients constitute a minority of those undergoing PCI.
Balloon angioplasty and coronary stenting are the mainstays of PCI. Other technologies include devices that ablate plaque (atherectomy), devices that remove clots from vessels (thrombectomy), and devices that capture and remove embolic debris (embolic protection).
IndicationsClass I indications for PCI are as follows[38] :
Patients with class II-IV angina or acute coronary syndrome with 1 or more significant lesions in 1 or more coronary arteries suitable for PCI Patients with acute ST-segment elevation myocardial infarction (STEMI) who can undergo angioplasty of the infarct artery within 12 hours of symptom onset or patients who have recurrent ischemia or infarction (rescue PCI) Patients older than 75 years who develop cardiogenic shock within 36 hours of an acute STEMIPatients with early ischemia (usually within 30 days) after CABGClass II indications for PCI are as follows:
Patients with focal saphenous vein graft (SVG) lesions or multiple stenosis who are poor candidates for reoperationThe presence of 1 or more lesions with reduced likelihood of success, or vessel or vessels subtending a less-than-moderate area of viable myocardium Patients with STEMI in whom thrombolytic therapy is contraindicatedPatients with STEMI who experience cardiogenic shock or hemodynamic instability after thrombolysisPatients with ischemia occurring 1-3 years postoperatively and preserved left ventricular function with discrete lesions in graft conduits Patients with disabling angina secondary to new disease in a native coronary circulationClass III indications for PCI are as follows:
Patients with no evidence of myocardial injury or ischemia on objective testing who have not undergone a trial of medical therapy, who have a small amount of salvageable myocardium, or who are at high risk of procedural success or morbidity or mortality Patients with insignificant coronary stenosisPatients with significant left main coronary artery disease (CAD) who are candidates for coronary artery bypass grafting (CABG)Patients who opt for elective PCI of a non–infarct-related artery at the time of myocardial infarction (MI)Patients with no evidence of myocardial ischemia after 12 hours of MI or routine PCI of the infarct artery after thrombolytic therapy Patients with total vein graft occlusionsA large multicenter cohort study evaluating data from patients 65 years or older who underwent elective PCI procedures suggests that same-day discharge can be considered for select patients who have low-risk clinical features, successful procedures without prolonged postprocedure use of parenteral antithrombotic agents, and adequate social support.[39]
Balloon angioplastyThe original technique of balloon angioplasty involved advancing a balloon-tipped catheter to an area of coronary narrowing, inflating the balloon, and then removing the catheter after deflation. Balloon angioplasty can reduce the severity of coronary stenosis, improve coronary flow, and diminish or eliminate objective and subjective manifestations of ischemia. To refine PCI techniques, medications and devices that replace or serve as adjuncts to the balloon catheter continue to be developed.
The mechanism of balloon angioplasty action involves 3 events: plaque fracture, compression of the plaque, and stretching of the vessel wall.[40] These lead to expansion of the external elastic lumina and axial plaque redistribution along the length of the vessel.
Coronary stentingCoronary stents are metallic scaffolds that are deployed within a diseased coronary artery segment to maintain wide luminal patency (see the first image below). They were devised as permanent endoluminal prostheses that could seal dissections, create a predictably large initial lumen, and prevent early recoil and late vascular remodeling so as to improve on both the early and the late results of balloon angioplasty (see the second image below).
 Metallic stent.
Metallic stent. 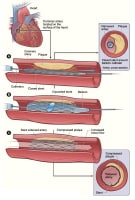 Deployment of stent in area of significant stenosis.
Deployment of stent in area of significant stenosis. Stents have become the most important development in interventional cardiology since the development of the balloon and the steerable guide wire systems. The first human coronary stent implants occurred in 1986 in France. The stent was an interwoven, helical, self-expanding design (Wallstent). A wire coil stent was used in 1987 in the United States. Stents were first applied to treat arteries that become acutely occluded after angioplasty.
Drug-eluting stents (DESs) elute medication to reduce restenosis within the stents (see the image below). Local release of rapamycin and its derivatives or of paclitaxel from a polymer matrix on the stent during the 30 days after implantation reduces inflammation and smooth muscle cell proliferation within the stent, decreasing in-stent late loss of luminal diameter from the usual 1 mm to as little as 0.2 mm. This dramatically lowers the restenosis rate after initial stent implantation or after secondary implantation of a DES for an in-stent restenosis.[41]
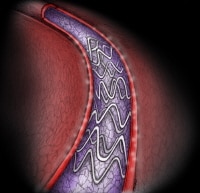 TAXUS Express paclitaxel-eluting coronary stent.
TAXUS Express paclitaxel-eluting coronary stent. A meta-analysis of 16 randomized trials suggested that sirolimus-eluting stents are superior to paclitaxel-eluting stents in terms of a significant reduction in the risk of reintervention and stent thrombosis. The risk of death was not significantly different between the 2 DESs, but there was a trend toward a higher risk of MI with paclitaxel-eluting stents, especially in the first year after the procedure.[42]
Everolimus is a derivative of sirolimus and has immunosuppressive and antiproliferative properties. The SPIRIT III trial randomly assigned 1002 patients with 1 or 2 de novo coronary artery lesions to everolimus-eluting low-profile, thin-strut stents or to paclitaxel-eluting polymer-based stents.[43] The everolimus stents yielded significantly better event-free survival rates at 2-year follow-up, with evidence of continued divergence of hazard curves for target vessel failure and major adverse cardiac events between 1 and 2 years.[43]
The ENDEAVOR II trial randomly assigned almost 1200 patients to the zotarolimus-eluting stent or the same bare-metal stent (BMS) without the drug; at 9 months, target lesion revascularization was significantly lower with the zotarolimus stent (4.6 vs 11.8%). The above 4 stents are presently the approved DESs in the United States, although additional DESs are in the pipeline.
In the ZEST trial, Park et al observed that zotarolimus-eluting stents had similar rates of major adverse cardiac events (ie, death, myocardial infarction, ischemia-driven revascularization) at 12 months as compared with sirolimus-eluting stents and lower rates of such events as compared with paclitaxel-eluting stents.[44]
Clinical trials have found DESs to be superior to BMSs for preventing in-stent restenosis and target vessel revascularization. In most studies, DESs reduced the need for repeat revascularization by more than 50% (to less than 5%) as compared with BMSs.[45, 46, 47] This is the chief reason for the reduction in major adverse cardiac events in the first 2 years after PCI in patients receiving DESs as opposed to BMS.[48] Clinical introduction of DESs correlates with a decline in referrals for CABG.[49]
Some reports have questioned the safety of DESs, suggesting that these devices are more frequently associated with stent thrombosis.[41, 50, 51] However, extended follow-up of patient cohorts to 4 years confirms the sustained benefit of DESs in decreasing the need for repeat revascularization without excess death or MI.[41, 52, 53]
A meta-analysis by Lee et al suggests that DESs have advantages over BMSs in SVG interventions.[54] In a comparison of the 2 stent types that involved 19 studies including 3,420 patients, target vessel revascularization was less frequently performed in patients who had undergone intervention with a DES, and the incidence of MI was lower. No differences were found in the risk of death or stent thrombosis.
Concern are still being raised that subsets of patients may be at increased risk for stent thrombosis if DESs are used in higher-risk off-label indications (eg, bifurcations, long lesions, small-diameter vessels, vein grafts, or restenotic disease). Hence, larger and longer follow-up studies of patients incorporating standard definitions of stent thrombosis and close monitoring of antiplatelet therapy compliance are needed.[55]
An analysis based on the database of the National Heart, Lung, and Blood Institute (NHLBI) Dynamic Registry concluded that even among patients with off-label indications, the use of DESs was not associated with a higher risk of death or MI than the use of BMSs but was associated with a lower rate of repeat revascularization at 1 year.[56] Various clinical trials have revealed that results of stent implantation depend on the patient’s risk profile, lesion characteristics, and other components of the coronary anatomy.
An important issue that remains to be resolved is the duration of dual antiplatelet therapy after DES implantation. Current guidelines recommend the administration of clopidogrel 75 mg/day to all post-PCI patients receiving a stent. Clopidogrel 75 mg/day should be given for at least 12 months if the patient is not at high risk for bleeding after receiving a DES.
For post-PCI patients receiving a BMS, clopidogrel should be given for a minimum of 1 month and ideally for as long as 12 months (unless the patient is at increased risk for bleeding, in which case the drug should be given for a minimum of 2 weeks). However, the optimal duration of clopidogrel therapy after 1 year has not been established and should depend on the risk–benefit ratio for the individual patient.
An open discussion about the need for and risks of dual antiplatelet therapy before placement of stents, especially DESs, should be undertaken by the physician. When patients are unwilling to or unable to comply with prolonged dual antiplatelet therapy, a BMS is preferred to a DES. See the images below.
Angioplasty versus stentingIn 1989, 2 randomized trials, the Stent Restenosis Study (STRESS) and the Belgium Netherlands Stent (BENESTENT) Study, compared stenting with balloon angioplasty, and their findings led to the approval of coronary stents.[57, 58] The superiority of stents in these trials was, at best, marginal. They reduced restenosis of the coronary artery without improving clinical outcome, at the cost of a higher rate of sudden coronary thrombosis and a marked increase in hemorrhagic complications resulting from drugs administered to prevent stent thrombosis.
Subsequent refinements in stent strategies improved clinical results dramatically. High-pressure poststent balloon inflations to provide complete expansion of stent devices[59] and replacement of warfarin by a thienopyridine (first ticlopidine, then clopidogrel) reduced stent thrombosis and hemorrhagic complications.
Compared with angioplasty, current BMSs reduce the frequency of restenosis and the need for a repeat revascularization procedure by approximately 50% (from 20-30% to 10-15%). Emergency coronary bypass rates have been brought down below 0.5%, coronary occlusion occurs less now with stents than it did with balloon angioplasty, and vascular complications arise less commonly than ever before. The improvement in outcomes with stents resulted in rapid transition from the traditional balloon angioplasty to PCI with stenting.
Currently, coronary stents are used in about 90% of interventional procedures. Stent-assisted coronary intervention has now replaced CABG as the most common revascularization modality in patients with coronary artery disease (CAD) and is used in patients with multivessel disease and complex coronary anatomy.
Other technologiesAtherectomy
The directional coronary atherectomy (DCA) catheter was first used in human peripheral vessels in 1985 and in coronary arteries in 1986.[60] In this procedure, a low-pressure positioning balloon presses a windowed steel housing up against the lesion; any plaque that protrudes into the window is shaved from the lesion by a spinning cup-shaped cutter and trapped in the device’s nose cone.
The Coronary Angioplasty Versus Excisional Atherectomy Trial (CAVEAT) demonstrated that atherectomy led to a higher rate of early complications, increased cost, and provided no apparent clinical benefit after 6 months of follow-up observation.[61] By 1994, stenting produced results similar to those of DCA, with fewer complications. Coronary DCA is rarely performed at present.
Rotational atherectomy uses a high-speed mechanical rotational stainless steel burr with a diamond chip–embedded surface. The burr is attached to a hollow flexible drive shaft that permits it to be advanced over a steerable guide wire with a platinum coil tip. The drive shaft is encased within a Teflon sheath through which flush solution is pumped to lubricate and cool the drive shaft and burr. A compressed air turbine rotates the drive shaft at 140,000-200,000 rpm during advancement across the lesion.
Rotational atherectomy is used in fewer than 5% of patients with PCI but is invaluable in patients with heavily calcified blockages, particularly those in which a balloon cannot be inflated.
Laser ablation
In laser ablation, an intense light beam travels via optical fibers within a catheter and enters the coronary lumen. After the target lesion is crossed with the guide wire, the laser catheter is advanced to the proximal end of the lesion. Blood and contrast medium are removed from the target vessel by flushing with saline before activating the laser. In view of their substantial cost and the lack of clinical benefit in comparison with other mechanical therapies, ablative laser techniques have not entered mainstream practice.
Mechanical thrombectomy
Intracoronary thrombi may be treated with mechanical thrombectomy devices (including rheolytic, suction, and ultrasonic thrombectomy devices). Although demonstrating the efficacy of such devices in randomized trials is difficult, dramatic results are often obtained in individual cases.
In rheolytic thrombectomy, high-speed water jets create suction via the Bernoulli-Venturi effect. The jets exit orifices near the catheter tip and spray back into the mouth of the catheter, creating a low-pressure region and intense suction. This suction pulls surrounding blood, thrombus, and saline into the tip opening and propels particles proximally through the catheter lumen and out of the body.
The catheters used for suction thrombectomy act via manual aspiration. They are advanced over a wire to the intracoronary thrombus, then passed through the thrombus while suction is applied to a hole in the catheter tip. Large intact thrombus fragments can be removed by means of this technique.
Ultrasonic thrombectomy involves the use of ultrasonic vibration to induce cavitation that can fragment thrombus into smaller components. The efficacy of this technique remains unproven and is under investigation.[62]
Embolization protection
Balloons and stents dislodge fragments of friable plaque or thrombus. Particle embolization appears to be one of the main causes of no-reflow and elevation of cardiac enzyme levels during SVG intervention.[63, 64] Several devices have been developed, undergone clinical trial evaluation, and gained regulatory approval for trapping such embolic material and removing it from the circulation (see the image below).
 Emboli captured during saphenous vein graft (SVG) intervention. FilterWire EZ system reduces major adverse events associated with SVG interventions.
Emboli captured during saphenous vein graft (SVG) intervention. FilterWire EZ system reduces major adverse events associated with SVG interventions. The Saphenous Vein Graft Angioplasty Free of Emboli Randomized (SAFER) trial was the first large trial that demonstrated the beneficial effect of protection devices on clinical outcomes.[65] Currently, use of these devices is a class I recommendation when PCI is being performed on SVGs.[38]
PreviousNextAdjunctive PharmacotherapyAspirinAspirin should be administered before undergoing any coronary revascularization procedure. Pretreatment with 75-325 mg of aspirin is recommended in patients undergoing either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). For long-term treatment, aspirin 75-325 mg/day is recommended. For long-term treatment after PCI in patients who receive antithrombotic agents such as warfarin, lower dosages (eg, 75-100 mg/day) are advised.[66]
ThienopyridinesPatients who undergo pretreatment with clopidogrel before PCI have better 30-day outcomes than patients who are not treated; the rate of death and myocardial infarction (MI) is reduced by nearly 39%.[67]
Currently, 12 months of dual antiplatelet therapy is recommended for all patients who receive a drug-eluting stent (DES)[68] unless there is a high risk of bleeding. The benefits and indications for treatment with dual antiplatelet therapy beyond 1 year in patients with a DES are the subject of ongoing studies. Low-dose aspirin should be continued indefinitely.
For patients with clinical features associated with stent thrombosis (eg, renal insufficiency, diabetes, or procedural characteristics such as multiple stents or treatment of a bifurcation lesion), extended dual antiplatelet therapy beyond 1 year may be reasonable. The risk of stent thrombosis must be balanced against the risk of other medical conditions and nonmedical factors that might affect the risk-benefit ratio of dual antiplatelet therapy as compared with other therapies.
Unfractionated heparinUnfractionated heparin (UFH) administered to achieve a target activated clotting time (ACT) longer than 200 seconds is recommended when a glycoprotein (GP) IIb/IIIa inhibitor is used. In patients who do not receive a GPIIb/IIIa inhibitor, the target ACT should be 250-350 seconds. Administration of additional anticoagulation to low-molecular-weight heparin depends on the timing of the last dose.
Low molecular weight heparinThe STEEPLE study[69] randomly assigned 3528 patients who underwent PCI to receive enoxaparin or UFH adjusted for ACT. In the setting of elective PCI, a single intravenous (IV) bolus of enoxaparin 0.5 mg/kg is associated with reduced rates of bleeding, and a dose of 0.75 mg/kg yields rates similar to those for unfractionated heparin, with more predictable anticoagulation levels.
Glycoprotein IIb/IIIa inhibitorsIn patients with acute coronary syndrome who are undergoing PCI without clopidogrel, a GPIIb/IIIa inhibitor in addition to heparin is a class I indication. Administration of these inhibitors is reasonable in patients with acute coronary syndrome who have undergone clopidogrel pretreatment and in those who have undergone elective PCI with stent placement. The greatest advantage of GPIIb/IIIa inhibitors is their ability to reduce periprocedural MI.
The 3 GPIIb/IIIa inhibitors are the following:
The monoclonal antibody abciximabThe nonpeptide tyrosine derivative tirofibanThe cyclic heptapeptide eptifibatideSignificant pharmacologic differences exist. For example, the platelet-bound half-life is long for abciximab and shorter for eptifibatide and tirofiban. The relative clinical efficacy of abciximab, tirofiban, and eptifibatide at the recommended doses is still uncertain.
Abciximab and tirofiban were compared in the TARGET trial, in which 4809 patients undergoing nonemergent stent-based PCI were randomly assigned to one agent or the other immediately before revascularization.[70] At 30 days after revascularization, tirofiban offered less protection from major ischemic events than abciximab did. However, by 6 months’ follow-up, there was no significant difference between the 2 drugs with respect to the combined endpoint or the individual endpoints.[71]
The only benefit from abciximab in TARGET appeared to be a reduction in procedure-related MI, primarily in patients with an acute coronary syndrome.[71] Although the exact reason for this is not known, a likely possibility is that more potent GPIIb/IIIa blockade occurred with abciximab at the doses used in the trial.
Direct thrombin inhibitorsThe Acute Catheterization and Urgent Intervention Triage Strategy Timing (ACUITY) trial revealed that bivalirudin plus a GPIIb/IIIa inhibitor was not inferior to heparin plus a GPIIb/IIIa inhibitor at 30 days. The Randomized Evaluation in PCI Linking Angiomax to Reduced Clinical Events (REPLACE-2) trial revealed that bivalirudin with provisional GPIIb/IIIa blockade was not inferior to heparin plus planned GPIIb/IIIa blockade during PCI and was associated with less bleeding.[72]
Therefore, bivalirudin can be used as an alternative to heparin both in patients who are not treated with GPIIb/IIIa antagonists and as an adjunct to GPIIb/IIIa antagonists in patients at high risk for bleeding.
In subgroups of patients with high-risk acute coronary syndrome (eg, the troponin-positive group), an increase in ischemic events was seen in the bivalirudin arm if subjects were not pretreated with clopidogrel. Bivalirudin can be used as an alternative to heparin in patients with acute coronary syndromes.
Even in patients with ST-segment elevation MI (STEMI) who are undergoing primary PCI, anticoagulation with bivalirudin alone, as compared with heparin plus GPIIb/IIIa inhibitors, was shown to result in significantly reduced 30-day rates of major bleeding and net adverse clinical events in the HORIZONS-AMI study.[73]
Parodi et al compared bivalirudin and UFH plus protamine in patients undergoing elective percutaneous transluminal coronary angioplasty (PTCA) who were pretreated with clopidogrel and aspirin; less major bleeding and fewer ischemic complications were observed in the patients who received bivalirudin than in those who received UFH plus protamine.[74]
PreviousNextComplicationsPercutaneous coronary interventionComplications of percutaneous coronary intervention (PCI) have been categorized as major (eg, death, myocardial infarction [MI], stroke, and emergency repeat revascularization)[75, 76] or minor (eg, transient ischemic attack, access-site complications[77] , renal insufficiency[78] , and adverse reactions to radiographic contrast).
Additional specific complications include intracoronary thrombosis, coronary perforation, tamponade, and arrhythmias. Bleeding is an increasingly worrisome complication because of the more common use of potent antithrombin and antiplatelet agents.[79] Late subacute thrombosis was observed in some series, particularly in patients who received drug-eluting stents (DESs).[80]
Coronary artery bypass graftingStroke and other neurologic complications are the most feared morbidity of coronary artery bypass grafting (CABG); the incidence of clinically obvious stroke is 0.8-5.2%.[81] Although coronary bypass surgery without cardiopulmonary bypass (CPB) may be associated with a lower risk of stroke, no randomized trial has demonstrated a lower incidence of stroke after off-pump surgery.
Deep sternal wound infection occurs in 1-4% of patients after CABG[82] and carries a mortality of nearly 25%. It is associated with obesity, diabetes, reoperation, use of both internal mammary arteries, and longer procedures.
Another common complication is postoperative renal dysfunction, defined as either a serum creatinine concentration of 2 mg/dL or higher or an increase of 0.7 mg/dL or more in comparison with preoperative values. MI can occur as a perioperative complication in 4-5% of patients.[83]
In-hospital mortality is approximately 1% for the lowest-risk elective patients and 2-5% for all patients. Risk varies in accordance with the presence or absence of comorbid conditions, the surgeon’s skill and experience, and the hospital’s volume of CABG procedures. Low albumin and age are strong predictors of adverse outcomes. Mortality may be as high as 20% if mitral valve surgery is needed in addition to CABG.[84]
PreviousNextOutcome ComparisonsRandomized clinical trials have assessed the outcomes of patients treated with medical therapy, percutaneous coronary intervention (PCI), and coronary artery bypass grafting (CABG). However, relatively few have been performed since the advent of drug-eluting stents (DESs).
CABG versus medical therapyBy the early 1980s, researchers had observed an improved quality of life, a decrease in the rate of myocardial ischemia, and an increase in the survival rate after CABG (compared with medical therapy) in selected subsets of patients (eg, those with left main coronary artery obstruction or 3-vessel disease, particularly those with a proximal left anterior descending (LAD) artery obstruction).[85]
In 1994, Yusuf et al[86] published a meta-analysis of 10-year results from randomized clinical trials in patients with stable angina, of whom 1324 were assigned to CABG and 1325 to medical treatment, and found that CABG conferred a survival benefit. The analysis demonstrated that a CABG improved survival compared with medical therapy (with crossover to surgery as needed) for patients with left main disease or 3-vessel disease with reduced ventricular function, and, possibly, 3-vessel disease with normal left ventricular function.
The meta-analysis showed that the CABG patients lived longer during a follow-up period of 10 years than those initially assigned to medical therapy.[86] A high crossover rate from medical therapy to surgery was observed (25% at 5 years and 41% at 10 years). Other data suggest that patients with 2-vessel disease with proximal LAD involvement may also derive a survival advantage with CABG.[87]
However, the medical therapy administered to patients randomized to the medical therapy arms of these trials predated the routine use of aspirin, beta blockers, statins, angiotensin-converting enzyme (ACE) inhibitors, angiotensin-receptor blockers, and calcium blockers, all of which improve survival, reduce angina, or both. Surgical therapy has also improved since these trials; the left internal mammary artery (LIMA) was used in only 14% of the patients enrolled in the trials.
With all of the improvements in medical and surgical therapy taken into account, CABG is still favored for patients with multivessel disease and reduced ventricular function. However, CABG has not been shown to be superior to complete revascularization with DESs.
Results of a randomized trial by Velazquez et al showed no significant difference in the death rate between patients with heart failure and coronary artery disease who underwent medical therapy alone and patients with the same conditions who underwent medical therapy plus CABG.[88]
In this study, 1212 patients with an ejection fraction of 35% or less and coronary artery disease (CAD) amenable to CABG were randomized to receive either medical therapy alone or medical therapy plus CABG. The primary outcome, death from any cause, occurred in 244 (41%) of the patients in the medical therapy group and 218 (36%) of those in the medical therapy plus CABG group. However, patients who underwent CABG, as opposed to those who received medical therapy only, had lower mortalities from cardiovascular causes.[88]
In a substudy of these patients, investigators assessed myocardial viability using single-photon emission computed tomography (SPECT), dobutamine echocardiography, or both; they were unable to identify a survival benefit from CABG as compared with medical therapy alone.[89]
The BARI 2D randomly assigned 2368 patients with both type 2 diabetes and heart disease to undergo either prompt revascularization with intensive medical therapy or intensive medical therapy alone and to undergo either insulin-sensitization or insulin-provision therapy. Primary endpoints were the rate of death and a composite of death, myocardial infarction, or stroke (major cardiovascular events).[90]
In the CABG stratum of this study (n = 763), the rate of major cardiovascular events was significantly lower in the revascularization group (22.4%) than in the medical-therapy group (30.5%). The death rate did not differ significantly between the revascularization group and the medical therapy group in either the CABG arm or the PCI arm.[90]
PCI versus medical therapyStable angina
The COURAGE trial[91] randomized 2287 patients with objective evidence of myocardial ischemia and stable CAD to an initial management strategy of either PCI plus optimal medical therapy or optimal medical therapy alone. PCI did not reduce the risk of death, myocardial infarction, or other major cardiovascular events when added to optimal medical therapy.
An initial strategy of PCI added to optimal medical therapy relieved angina and improved self-assessed health status to a greater extent than an initial strategy of optimal medical therapy alone for approximately 24 months. A greater benefit from PCI was observed in those patients with more severe and more frequent angina.[92]
Thus, patients with chronic CAD may expect relief from angina with either treatment strategy, and both strategies can have a profoundly positive effect on patients’ health status. The roles could be complementary, and some experts have suggested that optimal medical therapy might be considered first-line therapy, with PCI reserved for patients who do not have a response or who have severe baseline symptoms.
In COURAGE patients who underwent serial myocardial perfusion SPECT, adding PCI to optimal medical therapy resulted in greater reduction of ischemia than optimal medical therapy alone did. Rates of death or MI were directly proportional to the extent and severity of ischemia on the 6- to 18-month myocardial perfusion SPECT study.[93]
Acute coronary syndromes
A meta-analysis of 8 randomized controlled trials concluded that in acute coronary syndromes, an invasive strategy is beneficial in men and high-risk women for reducing the composite endpoint of death, MI, or rehospitalization with acute coronary syndrome. In contrast, the data supported a conservative strategy in low-risk women.[94]
In the guidelines published by the American College of Cardiology (ACC) and the American Heart Association (AHA), an early invasive strategy is indicated for those who have refractory angina or hemodynamic or electrical instability (without serious comorbidities or contraindications to such procedures) and in initially stabilized patients who have an elevated risk for clinical events.[95]
This recommendation is in accord with findings from other major contemporary trials (including FRISC-2,[96] TACTICS-TIMI18,[97] and RITA-3[98] ), which indicated that the greatest benefit from an invasive strategy was achieved in high-risk patients, particularly those with refractory angina, elevated cardiac troponin levels, dynamic ST-segment changes, and diabetes mellitus.
ST-segment elevation MI
The 2 types of reperfusion therapy are PCI capability and pharmacologic reperfusion. The DANish trial in Acute Myocardial Infarction (DANAMI) found that patients treated at facilities without interventional cardiology capabilities had better outcomes with transfer for PCI within 2 hours of presentation than with pharmacologic reperfusion treatment at the local hospital.[99]
However, when PCI capability is available, the best outcomes are achieved by offering this strategy 24 hours per day, 7 days per week.[100] In hospitals without PCI capability, immediate transfer for primary PCI is a treatment option when the expected door-to-balloon time is within 90 minutes of first medical contact.[101]
Better outcomes with primary PCI should not undermine the importance of fibrinolytic therapy. This is particularly relevant in that many hospital systems cannot consistently meet the time goal for primary PCI.[102] The aim with fibrinolytic therapy is to deliver the drug within 30 minutes of the time that the patient presents to the hospital. In these settings, transfer protocols need to be in place for arranging rescue PCI when clinically indicated.[103]
The fundamental concept is that faster times to reperfusion and better systems of care are associated with important reductions in morbidity and mortality rates in patients with ST-segment elevation MI (STEMI).
Another strategy that once appeared promising but has fallen out of vogue is facilitated PCI, a strategy of planned immediate PCI after an initial pharmacologic regimen (eg, full-dose fibrinolysis, half-dose fibrinolysis, a glycoprotein (GP) IIb/IIIa inhibitor, or a combination of reduced-dose fibrinolytic therapy and a platelet GPIIb/IIIa inhibitor). A planned reperfusion strategy using full-dose fibrinolytic therapy followed by immediate PCI is not recommended and is a class III recommendation according to the ACC/AHA guidelines.[104]
Facilitated PCI using regimens other than full-dose fibrinolytic therapy might be considered as a reperfusion strategy (class IIb recommendation) when the following are true:
Patients are at high riskPCI is not immediately available within 90 minutesBleeding risk is low (because of younger age, absence of poorly controlled hypertension, and normal body weight)The Occluded Artery Trial (OAT) tested the hypothesis that mechanical opening of a persistently occluded infarct-related artery at a time too late for myocardial salvage would improve long-term outcome.[105] It randomized patients to a strategy of medical therapy or routine PCI for total occlusion of the infarct-related artery 3-28 days after acute MI. The composite endpoint included death, reinfarction, or New York Heart Association (NYHA) class IV heart failure.
Contrary to the hypothesized benefit, the study showed high rates of procedural success with PCI and sustained patency but no clinical benefit with respect to death, reinfarction, or heart failure during an average 3-year follow-up.[105]
CABG versus PCISeveral clinical trials have compared CABG and percutaneous transluminal coronary angioplasty (PTCA), including the Coronary Angioplasty Versus Bypass Revascularization Investigation (CABRI), the Randomized Intervention Treatment of Angina (RITA) trial, the Emory Angioplasty versus Surgery Trial (EAST), the German Angioplasty Bypass Surgery Investigation (GABI), and the Bypass Angioplasty Revascularization Investigation (BARI).
Analyzed separately and together, these studies demonstrated that in patients with multivessel disease, the rates of death and MI during 1 to 10 years’ follow-up was similar when initial PTCA was compared with initial CABG. However, results of the 2 treatment modalities differed in that patients who underwent angioplasty required additional revascularization procedures much more commonly than did patients who underwent CABG because of the recurrence of symptoms (most commonly restenosis).
Patients who underwent CABG had longer initial hospitalizations and a longer period of convalescence but typically enjoyed greater relief of angina. A meta-analysis that compared 8 trials of CABG with angioplasty demonstrated no differences in hospital stay, mortality, or MI rate at 1 year[106] ; the main difference was in the number of repeat procedures.
Five randomized trials compared CABG with PCI in patients with multivessel disease in whom bare-metal stents (BMSs) were routinely placed during the PCI procedure. As in the CABG-versus-PTCA trials mentioned above, the frequencies of death and MI were similar in the 2 arms during the follow-up periods. Repeat revascularization was more common for those treated with stents than in those treated with CABG. The death and MI frequencies were half those observed in the older balloon angioplasty comparison trials.
In a meta-analysis of 23 randomized controlled trials,[107] 5019 patients randomly assigned to undergo PCI were compared with 4944 patients randomly assigned to undergo CABG. CABG was more effective than PCI in relieving angina and led to fewer repeated revascularizations but had a higher risk for procedural stroke. Survival to 10 years was similar for both procedures.
Although current ACC/AHA guidelines recommend CABG for the treatment of patients with unprotected left main CAD, that recommendation is based on clinical trials demonstrating a survival advantage for CABG as compared with medical therapy.
A meta-analysis by Lee et al suggests that PCI using a DES is safe in this patient population and could represent a good alternative to CABG in selected cases.[108] At 1-year follow-up in 2,905 patients from 8 clinical studies, no significant difference was noted in risk of death, MI, or stroke between the CABG and PCI groups, although the risk for target vessel revascularization was significantly lower in the CABG group.
Similarly, in 5-year results from the MAIN-COMPARE (Revascularization for Unprotected Left Main Coronary Artery Stenosis: Comparison of Percutaneous Coronary Angioplasty Versus Surgical Revascularization) registry, stenting and CABG showed similar rates of death and of the composite endpoint of death, Q-wave MI, or stroke in patients with unprotected left main CAD. Rates of target vessel revascularization were higher with stenting, however.[109]
Comparisons of current medical therapy, catheter intervention, and bypass surgery show that patients whose disease is well controlled by medical therapy do not gain a survival benefit from PCI or CABG and that those whose disease is not adequately controlled by optimal medical therapy have similar outcomes regardless of whether they undergo PCI or CABG.
An early invasive strategy is recommended for those patients who have refractory angina or hemodynamic or electrical instability or acute presentation and elevated risk for clinical events, preferably via quick access to a 24/7 intervention service.[110]
Enrollment is under way for several large randomized trials comparing CABG and DESs.
Previous, Comparison of Revascularization Procedures in Coronary Artery Disease





0 comments:
Post a Comment