An implantable cardioverter-defibrillator (ICD) is a specialized device designed to directly treat a cardiac tachydysrhythmia. ICDs have revolutionized the treatment of patients at risk for sudden cardiac death due to ventricular tachyarrhythmias. A permanent pacemaker is an implanted device that provides electrical stimuli, thereby causing cardiac contraction when intrinsic myocardial electrical activity is inappropriately slow or absent.
Essential update: New technique can eliminate imaging artifacts caused by ICDsRashid and colleagues developed a modified wideband late gadolinium enhancement (LGE) magnetic resonance imaging technique that can overcome hyperintensity image artifacts caused by implanted cardiac devices. In their study of 12 patients with ICDs, use of the wideband LGE sequence eliminated the severe, uninterpretable hyperintensity artifacts in the left ventricular wall that occurred with conventional LGE technique, thereby enabling confident evaluation of myocardial viability.[1]
Indications for ICD placementIndications for ICD implantation can be divided into 2 broad categories: secondary prophylaxis against sudden cardiac death and primary prophylaxis. For secondary prophylaxis, ICD placement is indicated as initial therapy in survivors of cardiac arrest due to VF or hemodynamically unstable VT. Published guidelines exclude cases in which there are “completely reversible causes,â€[2] although this exclusion is somewhat controversial.
Currently, indications for primary prophylaxis account for most of ICD implants, even though the evidence for such implants is often less well established. Class I indications (ie, the benefit greatly outweighs the risk, and the treatment should be administered) are as follows:
Structural heart disease, sustained VTSyncope of undetermined origin, inducible VT or VF at electrophysiologic study (EPS)Left ventricular ejection fraction (LVEF) LVEF ≤35%, NYHA class II or IIILVEF ≤30% due to prior MI, at least 40 days post-MILVEFClass IIa indications (ie, the benefit outweighs the risk and it is reasonable to administer the treatment) are as follows:
Unexplained syncope, significant LV dysfunction, nonischemic cardiomyopathySustained VT, normal or near-normal ventricular functionHypertrophic cardiomyopathy with 1 or more major risk factorsArrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) with 1 or more risk factors for sudden cardiac death (SCD)Long QT syndrome, syncope or VT while receiving beta-blockersNonhospitalized patients awaiting heart transplantBrugada syndrome, syncope or VTCatecholaminergic polymorphic VT, syncope or VT while receiving beta-blockersCardiac sarcoidosis, giant cell myocarditis, or Chagas diseasePacemaker indicationsAbsolute indications for pacemaker placement include the following:
Sick sinus syndromeSymptomatic sinus bradycardiaTachycardia-bradycardia syndromeAtrial fibrillation with sinus node dysfunctionComplete atrioventricular block (third-degree block)Chronotropic incompetence (inability to increase the heart rate to match a level of exercise)Prolonged QT syndromeCardiac resynchronization therapy with biventricular pacingRelative indications include the following:
Cardiomyopathy (hypertrophic or dilated)Severe refractory neurocardiogenic syncopeTemporary emergency pacing is indicated for therapy of significant and hemodynamically unstable bradydysrhythmias and for prevention of bradycardia-dependent malignant dysrhythmias.
Magnet InhibitionFeatures of magnet inhibition are as follows:
In most devices, placing a magnet over a permanent pacemaker temporarily "reprograms" the pacer into asynchronous mode; it does not turn the pacemaker off If the device company parameters are known, application of a magnet can determine whether the pacer's battery needs to be replaced Although many different branded pacemaker/ ICD magnets are available, in general, any pacemaker/ICD magnet can be used to inhibit the device Magnet use inhibits further ICD discharge; it does not, however, inhibit pacingIndications for ICD deactivation are as follows:
End-of-life care (after a discussion with the patient and family)Inappropriate shocksDuring resuscitationWith transcutaneous pacing (external pacing can cause an ICD to fire)During procedures such as central lines or surgery with electrocauteryICD complications and malfunctionsAcute surgical complications include the following:
PainBleedingPneumothoraxHemothoraxCardiac perforation with or without pericardial effusion and tamponade (sometimes requiring urgent drainage)Pulseless electrical activity following intraoperative defibrillation threshold testingSubacute ICD complications include the following:
PainInfectionPocket hematomaWound dehiscenceLead dislodgmentDeep venous thrombosisUpper extremity edemaDegradation of lead functionChronic complications include the following:
Device-related painLead fractureInappropriate shocksErosion of device through skinImmunologic rejection – RarePacemaker complications and malfunctionsPacemaker complications include the following:
PneumothoraxPericarditisInfectionSkin erosionHematomaLead dislodgmentVenous thrombosisMajor pacemaker malfunctions include the following:
Failure to outputFailure to captureFailure to sensePacemaker-mediated tachycardiaRunaway pacemakerPacemaker syndromeTwiddler's syndromeCardiac monitor pseudomalfunctionPacemaker pseudomalfunctionInpatient CareReasons for admission may include the following:
Device investigation: To determine if there is an eminent battery failure (multiple shocks will deplete battery life)Addition of antiarrhythmic medicationsTreatment of MI (which may be linked to the initial discharge)Treatment of patient discomfortProvision of psychological support: Up to 35% of people develop anxiety disorder following ICD placement[3] Image library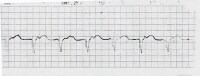 100% ventricular paced rhythm. NextOverview
100% ventricular paced rhythm. NextOverviewAn implantable cardioverter-defibrillator (ICD) is a specialized device designed to directly treat a cardiac tachydysrhythmia, while a permanent pacemaker is an implanted device that provides electrical stimuli, thereby causing cardiac contraction when intrinsic myocardial electrical activity is inappropriately slow or absent. A pacemaker senses intrinsic cardiac electric potentials, and, if these are too infrequent or absent, transmits impulses to the heart to stimulate myocardial contraction.
Newer-generation ICDs are also equipped with a demand pacing system and are a combination of an ICD and a pacemaker. (It is important to be aware that some older models [>10 years old] may lack this function.)
If a patient has a ventricular ICD and the device senses a ventricular rate that exceeds the programmed threshold, the device may elect to perform antitachycardia pacing or defibrillation. With antitachycardia pacing, the device fires a preset number of rapid pulses in succession in an attempt to terminate the ventricular tachycardia. If unsuccessful or if the rate falls in the preprogrammed cut of rate, the device will perform a cardioversion/defibrillation.
PreviousNextEvolution of the ICDThe implantable cardioverter-defibrillator (ICD) has revolutionized the treatment of patients at risk for sudden cardiac death due to ventricular tachyarrhythmias. Initially introduced in humans in 1980[4] and approved by the US Food and Drug Administration (FDA) in 1985, the ICD has evolved from a treatment of last resort to a first-line treatment and prophylactic therapy for patients at risk for ventricular tachycardia (VT) or ventricular fibrillation (VF).[2] Michel Mirowski conceived of and developed the ICD almost single-handedly. Prompted by the sudden death of a colleague, Mirowski conceived of an automatic, fully implantable defibrillator. Initially, lead systems were epicardial, requiring a thoracotomy for implantation, and pulse generators were large and bulky, requiring abdominal implantation.
Remarkable technologic advances have made ICDs easier and safer to implant and better accepted by patients and physicians. The development of transvenous lead systems, the addition of a second defibrillation coil in the superior vena cava, more effective biphasic defibrillation waveforms, and "active can" technology allows implantation in nearly all patients without the need for thoracotomy.[5]
Significant miniaturization of the capacitors and other components has reduced the size of the pulse generator tremendously, permitting subcutaneous pectoral implantation in most patients.[6, 7] A new generation of devices currently under development may be able to dispense with the transvenous lead system and still obtain satisfactory defibrillation, but without a capability for ventricular pacing.[8] On September 28, 2012, the U.S. Food and Drug Administration (FDA) approved the first subcutaneous implantable cardioverter defibrillator for ventricular tachyarrhythmias which allows the lead to be placed under the skin rather than through a vein into the heart.[9]
In addition to being considerably smaller than early generations of ICDs, current ICDs have markedly progressed in their therapeutic and diagnostic functions. Early devices were simple “shock boxes,†offering only high-energy shocks when the patient's heart rate exceeded a cut-off point. Diagnostic information was limited to the number of shocks delivered. Current devices offer tiered therapy with programmable antitachycardia pacing schemes, as well as low-energy and high-energy shocks in multiple tachycardia zones.
Dual-chamber, rate-responsive bradycardia pacing is now available in all ICDs, and sophisticated discrimination algorithms minimize shocks for atrial fibrillation, sinus tachycardia, and other non–life-threatening supraventricular tachyarrhythmias. Diagnostic functions, including stored electrograms, allow for verification of shock appropriateness. Device battery longevity has also increased; early devices lasted 2 years or less, while current devices are expected to last 6 years or longer.
PreviousNextICD Clinical TrialsEarly data regarding the implantable cardioverter-defibrillator (ICD) was drawn primarily from uncontrolled series of patients for whom antiarrhythmic drug therapy for ventricular tachycardia(VT) or ventricular fibrillation (VF) had failed. Even in these refractory patients, initial series suggested a markedly reduced risk of sudden, presumed arrhythmic, death. Subsequent randomized, controlled trials also focused on secondary prevention of sustained VT, VF, and sudden cardiac death.[10] In the early to-mid 1990s, 3 clinical trials were conducted in patients who had survived life-threatening ventricular tachyarrhythmias.
The Antiarrhythmics Versus Implantable Defibrillators (AVID) trial, conducted in the United States, enrolled patients with prior cardiac arrest or hemodynamically significant sustained VT and randomized patients to either ICD implantation or antiarrhythmic drug therapy, including primarily amiodarone and, in a few cases, sotalol.[11] The Canadian Implantable Defibrillator Study (CIDS) trial in Canada had a similar structure.[12, 13] The Cardiac Arrest Study Hamburg (CASH) trial in Hamburg enrolled cardiac arrest survivors and randomized them to amiodarone, metoprolol, propafenone, or ICD implantation.[14]
The AVID trial, although sponsored by the National Institutes of Health (NIH), was extremely controversial, with many electrophysiologists protesting that a randomized trial was not necessary to prove the effectiveness of the ICD. This trial was terminated prematurely because of improved survival rates in the ICD-treated patients. The CIDS trial yielded similar results. In the CASH trial, the propafenone arm of the study was terminated prematurely due to an increased mortality rate.[15] Ultimately, in the CASH trial, ICD therapy proved superior when compared with either amiodarone or metoprolol therapy.
These trials firmly established the ICD as preferred therapy in patients who have survived cardiac arrest or hemodynamically significant, sustained VT.
Subgroup analyses of AVID, CIDS, and the primary prevention Multicenter Automatic Defibrillator Implantation Trial[16] suggested that the survival benefit of the ICD is realized primarily by the patients who are sicker; ie, those with greater impairment of left ventricular systolic function, as measured by left ventricular ejection fraction (LVEF). In these 3 trials, the ICD had less apparent benefit in patients with better-preserved left ventricular systolic function.
Several important trials have subsequently been performed examining the role of ICDs as primary therapy for patients who are at risk for but who have not yet manifested sustained ventricular arrhythmias. These trials include the MADIT (Multicenter Automatic Defibrillator Implantation Trial), MUSTT (Multicenter Unsustained Tachycardia Trial), MADIT II, SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial), and the COMPANION (Comparison of Medical Therapy, Pacing, and Defibrillation in Chronic Heart Failure) trial.
MADIT studySimilar to the AVID trial, MADIT was terminated prematurely because of a significant survival benefit seen in patients treated with ICDs. MADIT in 1996 had enrolled patients with ischemic cardiomyopathy (LVEF ≤35%) and asymptomatic, nonsustained VT who had inducible sustained VT or VF not suppressible with procainamide infusion during electrophysiology study (EPS). Enrolled patients were randomized to either ICD implantation or to therapy considered appropriate by the treating physician. Antiarrhythmic drug therapy was administered in both arms as considered appropriate by the treating physician.[16]
MUSTTMUSTT, reported in 1999, also showed a survival benefit to ICD therapy. The trial had similar inclusion criteria to MADIT (prior infarct, LVEF ≤40%, nonsustained VT inducible at EPS) and randomized patients to EPS-guided therapy versus no specific antiarrhythmic therapy.
Early in the trial, EPS-guided therapy consisted of antiarrhythmic drug therapy guided by EPS testing, with ICD implantation reserved for patients with ventricular arrhythmias refractory to antiarrhythmic drugs. Later in the trial, ICDs were used earlier in patients who were randomized to the EPS-guided therapy arm of the trial. MUSTT showed a survival benefit in the EPS-guided group. The survival benefit was attributable to the ICD. Patients who were randomized to EPS-guided therapy and treated with antiarrhythmic drugs fared no better or worse than patients assigned to the control arm of the trial.[17]
In MUSTT, a registry was maintained of patients who met the clinical criteria for the study but were noninducible in the electrophysiology laboratory. During follow-up, the survival rate in this group was better than in the inducible patients assigned to the control group but not as good as in inducible patients who received ICDs. Although MUSTT was not designed to determine the optimal treatment in noninducible patients, many have concluded that, in the population studied, EPS testing may be used to stratify high-risk and moderate-risk patients rather than high-risk and low-risk patients.[18, 19]
MADIT IIThe Multicenter Automatic Defibrillator Implantation Trial II (MADIT II) in 2002 markedly expanded the potential pool of ICD recipients. MADIT II randomized patients with prior myocardial infarction and LVEF at or below 30% to ICD therapy or a control group. Nonsustained VT or inducible VT at EPS was not required. Patients who received an ICD had a 31% reduction in mortality rate. An important aspect of MADIT II was that subjects in both arms of the trial were well managed medically with a high rate of beta-blocker, angiotensin-converting enzyme (ACE) ̶ inhibitor, and cholesterol-lowering medication usage.[20]
Information regarding cost implications of ICDs continues to emerge. The MADIT II study showed that prophylactic implantation of a defibrillator reduced the rate of mortality in patients with a previous myocardial infarction and low LVEF. The cost analysis phase of the study showed that during the 3.5-year period of the study, the average survival gain for the defibrillator arm was 0.167 years (2mo), the additional costs were $39,200, and the incremental cost-effectiveness ratio (iCER) was $235,000 per year-of-life saved. In 3 alternative projections to 12 years, this ratio ranged from $78,600 to $114,000. Estimated cost per life-year saved is relatively high at 3.5 years, but projected costs are substantially lower over the course of longer time horizons.[21]
COMPANION trialIn the COMPANION trial, pacemakers and, to an even greater extent, ICDs, were found to reduce the risk of death in patients with advanced heart failure, even when there was no indication for pacemaker or ICD treatment.
In the COMPANION trial, patients with advanced heart failure, New York Heart Association (NYHA) functional class III or IV, an LVEF of 35% or less, and intraventricular conduction delay with QRS duration of over 120 milliseconds, but with no indication for pacemaker or ICD implant, were randomized to optimal medical therapy alone or in combination with cardiac resynchronization therapy with either a biventricular pacemaker or biventricular pacemaker-defibrillator.
Risk of hospitalization or death from heart failure was reduced by 34% in the pacemaker group and by 40% in the defibrillator group. Risk of death from any cause was reduced by 24% in the pacemaker group and by 36% in the defibrillator group.[22]
SCD-HeFTIn SCD-HeFT, a primary prevention trial reported in 2005 in which subjects with an LVEF of 35% or less and symptoms in NYHA functional class II or III were randomly assigned to 1 of 3 treatment groups—conventional heart failure therapy plus placebo, conventional heart failure therapy plus amiodarone, or conventional heart failure therapy plus ICD implant—ICD therapy, as compared with placebo, was associated with a 23% reduction in the risk of death from any cause and an absolute 7% decrease in mortality over 5 years.
No difference in mortality benefit was shown between subjects with ischemic cardiomyopathy (70% of enrollees) and those with nonischemic cardiomyopathy. ICD therapy benefited only NYHA functional class II subjects.[23]
Other studiesA meta-analysis of 5 clinical trials that included 4317 patients with NYHA functional class I/II heart failure performed by Adabag et al suggested that asymptomatic patients with NYHA functional class I/II heart failure may benefit from cardiac resynchronization therapy.[24] This therapy has been shown to decrease all-cause mortality, reduce heart failure hospitalizations, and improve LVEF in these patients. The authors did caution that risks versus benefits do need to be taken into consideration for this group of patients.
Levy et al found that in patients with moderately symptomatic heart failure with an ejection fraction of 35% or less, primary prevention with an ICD provides no benefit in some cases but substantial benefit in others, and that ICD benefit can be predicted. Analysis of data from the placebo arm of SCD-HeFT showed that patients could be classified into 5 groups on the basis of predicted 4-year mortality. In the treatment arm, ICD implantation decreased relative risk of sudden cardiac death by 88% in patients with the lowest baseline mortality risk, versus 24% in the highest-risk group. ICD treatment decreased relative risk of total mortality by 54% in the lowest-risk group but provided no benefit (2%) in the highest-risk group.[25]
Women enrolled in primary prevention ICD trials have had the same mortality compared with men, while experiencing significantly fewer appropriate ICD interventions, thus suggesting a smaller impact of sudden cardiac death on overall mortality in women with dilated cardiomyopathy.[26]
PreviousNextTrials Showing No Benefit From ICD TherapyAt least 4 notable, published implantable cardioverter-defibrillator (ICD) trials have failed to demonstrate a significant survival benefit to ICD therapy over optimal medical therapy. Two of these trials examined patients with ischemic cardiomyopathy and 2 examined patients with nonischemic cardiomyopathy.
DEFINITEThe Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation Trial (DEFINITE) enrolled subjects with nonischemic cardiomyopathy and showed a trend toward mortality benefit in the ICD arm, with mortality at 2 years being 14.1% in the medical therapy arm and 7.9% in the ICD arm.[27]
CATThe Cardiomyopathy Trial (CAT), in which 104 subjects with recent ([28]
CABG-Patch trialIn the Coronary Artery Bypass Graft (CABG)-Patch trial, ICD implantation improved the sudden cardiac death mortality rate but not the total mortality rate. In the study, subjects undergoing CABG who had decreased left ventricular function (ejection fraction [29] The lack of effectiveness of IDCs on the mortality rate apparently resulted from the poor predictive value of a preoperative SAECG in identifying patients at risk for arrhythmic death or the salutatory effects of coronary revascularization in reducing the risk of arrhythmic death.
DINAMITIn the Defibrillator in Acute Myocardial Infarction Trial (DINAMIT)—which enrolled subjects within 40 days of an acute myocardial infarction and randomized them to optimal medical therapy with or without a defibrillator—no difference was shown in mortality at a mean follow-up of 2.5 years. The CABG-Patch and DINAMIT trials each involved a possible confounder of revascularization.[30]
AMIOVIRTA small trial that directly compared ICD therapy with medical therapy of amiodarone found no mortality difference between the 2 treatments at 3 years. The Amiodarone Versus Implantable Cardioverter-Defibrillator Randomized Trial (AMIOVIRT) studied 103 subjects with nonischemic cardiomyopathy and was stopped early when a prespecified rule for futility was reached.[31] (The SCD-HeFT trial provided only an indirect comparison between amiodarone therapy and ICD therapy because the trial was designed only to compare each of these therapies individually with optimal medical therapy.)[23]
CRT-D treatment studyA study by Barsheshet et al suggested that patients with ischemic cardiomyopathy have a higher risk of heart failure or death that is directly related to cardiac resynchronization therapy with defibrillator (CRT-D) and the elapsed time from myocardial infarction.[32]
ICD treatment post-myocardial infarctionIn the case of post-myocardial infarction (MI) patients, no mortality benefit was observed from placing ICDs in patients with reduced ejection fraction until after 40 days post-MI and patient reassessment. This is likely due to the fact that death in the first 40 days post-MI may be attributed to causes other than arrhythmia.[33]
Age-related studiesSome data suggest that not all age groups benefit equally from the protective effect of ICDs. One pooled analysis of primary prevention trials found that the elderly do not derive a clinically significant benefit from ICDs.[34] Moreover, there was an overall 17% complication rate associated with ICD treatments. In this analysis, elderly was defined as older than 60 for some studies and older than 65 for others.
A pooled analysis of secondary prevention ICD trials also found no reduction in all-cause and arrhythmic mortality in elderly patients.[35] However, current recommendations do not exclude ICD implantation on the basis of age.
PreviousNextICD IndicationsIndications for implantable cardioverter-defibrillator (ICD) implant can be divided into 2 broad categories: secondary prophylaxis against sudden cardiac death and primary prophylaxis. Multiple studies have shown the ICD to be superior to antiarrhythmic drug therapy in patients with a history of life-threatening VT and VF. Therefore, the indications for secondary prophylaxis are well supported by clinical evidence gained from randomized clinical trials.[2] Currently, however, indications for primary prophylaxis account for most of ICD implants, even though the evidence for such implants is often less well established.
Investigators have looked at ongoing indications for ICD therapy at the time of elective device replacement. In one prospective cohort study, 21% of patients received appropriate ICD therapy within 3 years following device replacement, even if they had never received appropriate therapy from their originally implanted device. For patients who had received appropriate therapy from their first device, 48% received appropriate therapy over the same 3-year period.[36]
Secondary prophylaxisAn ICD is recommended as initial therapy in survivors of cardiac arrest due to VF or hemodynamically unstable VT. Published guidelines exclude cases in which there are “completely reversible causes.â€[2]
The exclusion for completely reversible causes is somewhat controversial. As an example, an acute MI predisposes to polymorphic VT, and the culprit lesion may be reversed with intracoronary stenting. However, we know that any patient who presents with an MI is at increased risk of recurrent MI, which may again precipitate an unstable ventricular arrhythmia. One school of thought suggests that such patients should undergo ICD implant, even though the cause of cardiac arrest is completely reversible, because the risk of recurrence is increased.
In another example, consider cardiac arrest secondary to transient prolongation of the QT interval, perhaps secondary to drug therapy. QT interval prolongation increases the risk of torsades de pointes, a potentially life-threatening arrhythmia. Withdrawal of the offending agent may normalize the QT interval, thereby reversing the cause of cardiac arrest. However, such a patient remains at risk of recurrent QT prolongation and subsequent cardiac arrest, perhaps from an electrolyte disturbance or as a result of ingestion of a different QT-prolonging agent.
Primary prophylaxisIndications for an ICD implant as primary prophylaxis against sudden cardiac death are listed in the Table 1, below. The indications are listed as Class I or Class IIa, as classified by the ACC/AHA 2008 guidelines. Class I means that the treatment is useful, that its benefit greatly outweighs the risk, and that it should be administered.
Class IIa means that the benefit outweighs the risk and it is reasonable to administer the treatment. Class IIb means that the benefit probably outweighs the risk and that the treatment may be considered. Class III means that the risk outweighs the benefit, and the treatment should not be performed. Only Class I and Class IIa indications are included in the table. For a complete list, the reader is referred to the American College of Cardiology (ACC)/American Heart Association (AHA) 2008 guidelines.[2]
The greatest predictors of risk for sudden cardiac death include left ventricular systolic function and heart failure symptoms. The vast majority of investigational studies have quantified left ventricular systolic function using the measure of left ventricular ejection fraction (LVEF). The most widely used form of heart failure symptom classification is the New York Heart Association (NYHA) functional class classification system, which classifies mild to no symptoms as Class I, and the most severe symptoms as Class IV.
Table 1. Indications for ICD Implant (Open Table in a new window)
Indication Classification Supporting Studies Structural heart disease, sustained VTClass IAVID, CASH, CIDSSyncope of undetermined origin, inducible VT or VF at EPSClass ICIDSLVEF Class ISCD-HeFTLVEF ≤35%, NYHA Class II or IIIClass ISCD-HeFTLVEF ≤30% due to prior MI, at least 40 days post-MIClass IMADIT IILVEF Class IMADIT, MUSTTUnexplained syncope, significant LV dysfunction, nonischemic CMClass IIaExpert opinionSustained VT, normal or near-normal ventricular functionClass IIaExpert opinionHypertrophic CM with 1 or more major risk factorsClass IIaExpert opinionArrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) with 1 or more risk factors for sudden cardiac death (SCD)Class IIaExpert opinionLong QT syndrome, syncope or VT while receiving beta blockersClass IIaZareba et al[37] , Viskin et al[38] , Goel et al[39] , Monnig et al[40] , Goldenberg et al[41] , Hobbs et al[42] Nonhospitalized patients awaiting heart transplantClass IIaExpert opinionBrugada syndrome, syncopeClass IIaExpert opinionBrugada syndrome, VTClass IIaExpert opinionCatecholaminergic polymorphic VT, syncope or VT while receiving beta blockersClass IIaExpert opinionCardiac sarcoidosis, giant cell myocarditis, or Chagas diseaseClass IIaExpert opinionPreviousNextPacemaker IndicationsAbsolute indications for pacemaker placement include the following:
Sick sinus syndromeSymptomatic sinus bradycardiaTachycardia-bradycardia syndromeAtrial fibrillation with sinus node dysfunctionComplete atrioventricular block (third-degree block)Chronotropic incompetence (inability to increase the heart rate to match a level of exercise)Prolonged QT syndromeCardiac resynchronization therapy with biventricular pacingRelative indications include the following:
Cardiomyopathy (hypertrophic or dilated)Severe refractory neurocardiogenic syncopeTemporary emergency pacing is indicated for therapy of significant and hemodynamically unstable bradydysrhythmias and for prevention of bradycardia-dependent malignant dysrhythmias. Examples include refractory symptomatic sinus node dysfunction, complete heart block (see the image below), alternating bundle-branch block, new bi-fascicular block, and bradycardia-dependent ventricular tachycardia.
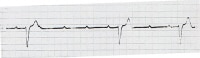 Complete heart block
Complete heart block Examples of indications for prophylactic temporary pacing include insertion of a pulmonary artery catheter in a patient with an underlying left bundle-branch block, use of medications that may cause or exacerbate hemodynamically significant bradycardia, prophylaxis during the perioperative period surrounding cardiac valvular surgery, Lyme disease or other infections (Chagas disease) that cause interval changes, and prolonged PR intervals.
PreviousNextDevice InsertionPacing systems consist of a pulse generator and pacing leads. With permanent systems, endocardial leads are inserted transvenously and advanced to the right ventricle and/or atrium, where they are implanted into the myocardial tissue. The pulse generator is placed subcutaneously or submuscularly in the chest wall.
Pulse generators contain a battery, as well as sensing, timing, and output circuits. The battery (most commonly lithium-iodide) typically has a lifespan of 5-10 years. Pulse generators can be set to fixed-rate (asynchronous) or demand (synchronous) modes. In the asynchronous mode, impulses are produced at a set rate independent of intrinsic cardiac activity. This mode carries a small, but inherent, danger of producing lethal dysrhythmias should the impulse coincide with the vulnerable period of the T wave. In the synchronous mode, the sensing circuit searches for an intrinsic depolarization potential. If this is absent, a pacing response is generated. This mode closely mimics intrinsic myocardial electrical activity.
During pacemaker placement, signal amplitude and width are set high enough to reliably achieve myocardial capture, yet low enough to maximize battery life.
Temporary systems use an external pulse generator with leads placed either transcutaneously or transvenously. Transcutaneous leads are the easiest and most convenient to use for rapid application of temporary pacing and is the method of choice during emergency department (ED) resuscitation. Transcutaneous pacing may be uncomfortable, and patients may require mild sedation (eg, benzodiazepine). Once the patient is stabilized or central venous access is gained, transvenous leads provide the most reliable and comfortable pacing mechanism and are a good transition to permanent systems.
For transvenous temporary pacing, semirigid catheters are inserted through a central venous access. Electrocardiographic monitoring (specifically V1) is used to track catheter positioning. For example, P-wave morphology is initially inverted and becomes upright as the catheter is in line with the SA node. QRS morphology is also initially inverted, transitioning to isoelectric and then upright as the tip is placed in the apex. An injury pattern resembling ST elevation ensures that the catheter tip is in proper positioning for pacing. Semifloating or flexible balloon-tipped catheters can be used in emergencies since they may be positioned without such monitoring.
Many patients who undergo ICD or pacemaker implant are anticoagulated with warfarin. A strategy of implanting devices during uninterrupted warfarin therapy appears to have a lower bleeding risk than a strategy of temporarily discontinuing warfarin and bridging with heparin.[43] We await similar data for newer anticoagulants such as dabigatran and rivaroxaban.
PreviousNextPacing CodesThe Heart Rhythm Society and the British Pacing and Electrophysiology Group (BPEG) have developed a code to describe various pacing modes. (See Table 2, below.)[44]
Table 2. Pacemaker Code Used to Describe Various Pacing Modes (Open Table in a new window)
1st Position 2nd Position 3rd Position 4th Position 5th Position ChamberPaced
Chamber
Sensed
Response to
Sensing
Rate ModulationMultisite PacingAATOOVVIRADDDVOODAbbreviations: A, atrium; V, ventricle; D, dual (both chambers); O, none; T, triggered; I, inhibited; R, rate adaptive.Pacing code explanation:
A typical pacing code consists of 3-5 letters. The first letter indicates the chamber(s) paced, as follows:
A - Atrial pacingV - Ventricular pacingD - Dual-chamber (atrial and ventricular) pacingThe second letter indicates the chamber in which electrical activity is sensed, as follows:
A, V, or DO is used when pacemaker discharge is not dependent on sensing electrical activity.The third letter refers to the response to a sensed electric signal, as follows:
T - Triggering of pacing functionI - Inhibition of pacing functionD - Dual response (ie, any spontaneous atrial and ventricular activity will inhibit atrial and ventricular pacing, and lone atrial activity will trigger a paced ventricular response) O - No response to an underlying electric signal (usually related to the absence of associated sensing function)The fourth letter represents rate modulation, as follows:
R - Rate-response ("physiologic") pacingO - No programmability or rate modulationThe fifth letter represents multisite pacing, as follows:
A - AtrialV - VentricularD - Dual (pacing + shock)Although the first 3 letters of the pacing code are used most commonly, a 5-position code is currently in use.
Modern pacemakers have multiple functions. The simplest settings are VVI and AAI. The VVI mode senses and paces the ventricle and is inhibited by a sensed ventricular event. Alternatively, the AAI mode senses and paces in the atrium, and each sensed event triggers the generator to fire within the P wave. (See the image below.)
 100% ventricular paced rhythm.
100% ventricular paced rhythm. The most common setting, the DDD mode, denotes that both chambers are capable of being sensed and paced. This requires 2 functioning leads, one in the atrium and the other in the ventricle. On the electrocardiogram (ECG), if both atrium and ventricle are being paced, there will be a pacing artifact before the P wave and preceding the QRS. The first pacing artifact indicates the atrial depolarization, and the second indicates the initiation of the QRS complex. Given that one of the leads is in the right ventricle, a left bundle-branch pattern may be evident on ECG.
Note that a 2-wired system does not necessarily need to be in DDD mode, since the atrial or ventricular leads can be programmed off. Additionally, single tripolar lead systems are available that can sense atrial impulses and either sense or pace the ventricle. Thus, this system provides for atrial tracking without the capability of atrial pacing and can be used in patients with AV block and normal sinus node function.
Pacemaker programming can be performed noninvasively by an electrophysiology technician or cardiologist. Because of the myriad of pacemaker types, patients should carry a card with them providing information about their particular model. Most pacemaker generators have an x-ray code that can be seen on a chest radiograph; however, the chest radiography may need to be zoomed onto the pacemaker generator for better resolution. The markings, along with the shape of the generator, may assist with deciphering the manufacturer of the generator and pacemaker battery.
For further information or locations of technicians for pacemaker devices, the device company can be contacted at the following 24-hour help-line telephone numbers below[45] :
Guidant (Boston Scientific) - 800-CARDIAC (800-227-3422)Medtronic - 800-MEDTRONIC (800-633-8766)St. Jude Medical - 800-722-3774Biotronik - 800-547-0394PreviousNextMagnet InhibitionIn most devices, placing a magnet over a permanent pacemaker temporarily "reprograms" the pacer into asynchronous mode; it does not turn the pacemaker off. Each pacemaker type has a unique asynchronous rate for beginning of life (BOL), elective replacement indicator (ERI), and end of life (EOL). Therefore, if the device company parameters are known, application of a magnet can determine if the pacer's battery needs to be replaced. Further interrogation or manipulating of the device should be performed by an individual skilled in the technique.
Although many different branded pacemaker/implantable cardioverter-defibrillator (ICD) magnets are available, emergency physicians should be aware that, in general , any pacemaker/ICD magnet can be used to inhibit the device.
It is worth mentioning that, when a magnet is applied to an ICD, it can temporarily turn off defibrillation therapy without altering its pacing ability.
Also, note that the majority of devices have a magnet response; however, some devices can be programmed to not respond to magnet application and thus will need a device programmer to change the parameters.
Magnet use inhibits further ICD discharge. It does not, however, inhibit pacing. In some devices, application of a magnet produces a soft beep for each QRS complex. If the magnet is left on for approximately 30 seconds, the ICD is disabled and a continuous tone is generated. To reactivate the device, the magnet must be lifted off the area of the generator and then replaced. After 30 seconds, the beep returns for every QRS complex. Indications for ICD deactivation are as follows:
End-of-life care (after a discussion with the patient and family)Inappropriate shocksDuring resuscitationWith transcutaneous pacing (external pacing can cause an ICD to fire)During procedures such as central lines or surgery with electrocauteryPreviousNextAdjunctive Care in ICD TherapyAlthough implantable cardioverter-defibrillators (ICDs) are extremely effective in terminating life-threatening arrhythmias, many patients require adjunctive therapy to reduce the frequency of arrhythmic events that require therapy. This generally consists of pharmacologic therapy, and, particularly in cases of failure of drug therapy, radiofrequency catheter ablation.
Inappropriate shocks may be delivered for atrial fibrillation, sinus tachycardia, and other types of supraventricular tachycardia, prompting ICD reprogramming or adjunctive therapy.
See The Heart Rhythm Society's Expert Consensus Statement on the Management of Cardiovascular Implantable Electronic Devices (CIEDs) in Patients Nearing End of Life or Requesting Withdrawal of Therapy.[46]
PreviousNextICD Complications and MalfunctionsSeveral complications of implantable cardioverter-defibrillators (ICD) implant have been described, some of which are currently tracked in a national database of ICD implants. Acute surgical complications include the following:
PainBleedingPneumothoraxHemothoraxCardiac perforation with or without pericardial effusion and tamponade (sometimes requiring urgent drainage)Pulseless electrical activity following intraoperative defibrillation threshold testingAn analysis of more than 350,000 ICD implantations included in the National Cardiovascular Data Registry–ICD Registry revealed 3.1% of patients experienced inhospital adverse events, 1.2% experienced major adverse events, and 0.4% died. Adverse events were lower (1.9%) with single-chamber ICD implants than with dual-chamber ICD implants (2.9%) or with biventricular ICD implants (4.1%). Specific adverse event rates included lead dislodgement (1%), hematoma (0.9%), pneumothorax (0.4%), and cardiac arrest (0.3%).
Physician level of training and level of specialty certification have been shown to affect the risk of adverse events associated with ICD implant. An ICD Registry analysis found that physicians who implant more ICDs have lower rates of procedural complications and inhospital mortality[47] Implant volume may partially explain the difference in adverse events among physicians with different specialty certifications. However, no inverse relationship was found between procedure volume and adverse event rate observed within the board certified category.
Subacute and chronic complicationsSubacute ICD complications include the following:
PainInfectionPocket hematomaWound dehiscenceLead dislodgmentDeep venous thrombosisUpper extremity edemaDegradation of lead functionChronic complications include the following:
Device-related painLead fractureInappropriate shocksErosion of device through skinImmunologic rejection - RareInfectionICD infection rates are higher in patients undergoing generator replacement compared with de novo implant.[48] A prospective study revealed an infection rate of 1.3% in patients undergoing device replacement.[49] In this study, postoperative hematoma significantly increased the risk of infection (22.7% vs 0.98%).
Inappropriate shocksOne of the risks of ICD implant is that of inappropriate ICD shocks. An inappropriate ICD shock is one that is not precipitated by accurate detection of a malignant ventricular arrhythmia, VT, or VF.[50] Typically, inappropriate ICD shocks result when atrial arrhythmias, such as atrial fibrillation, atrial tachycardia, or atrial flutter, accelerate the ventricular rate beyond the set limit for delivery of ICD shock therapy.
However, inappropriate shocks may also result from sinus tachycardia, supraventricular tachycardia (SVT), illicit drug use (as with cocaine and methamphetamine), and ventricular oversensing. Ventricular oversensing may occur due to T-wave oversensing, electromagnetic interference (EMI), a loose setscrew in the ICD header, or ICD lead fracture.
Analysis of the MADIT II trial data revealed that 11.5% of the ICD patients received inappropriate ICD shocks and that 31.2% of all ICD shocks were deemed inappropriate. Inappropriate ICD shocks were attributed to atrial fibrillation (44%), supraventricular tachycardia (36%), and abnormal sensing (20%). Patients with inappropriate shocks had greater all-cause mortality.[51]
Drug therapy with hydroxymethylglutaryl-coenzyme A reductase inhibitors, or so-called statins, has been shown to reduce, by more than half, the frequency of inappropriate ICD shocks secondary to occurrence of atrial fibrillation and atrial flutter.[52]
There is some indirect evidence that the incidence of inappropriate shocks may be lower in patients with dual-chamber devices compared with patients who receive single-chamber devices.[53]
Failure to shock and ineffective cardioversionFailure to deliver a shock may be caused by failure to sense, lead fracture, EMI, and inadvertent ICD deactivation. Management includes external defibrillation or cardioversion and antidysrhythmic medications.
Ineffective cardioversion may result from inadequate energy output, rise in defibrillation threshold (possibly due to an antiarrhythmic medication, such as amiodarone, flecainide, or phenytoin), myocardial infarction at the lead site, lead fracture, insulation breakage, scarring at the lead implantation site, and lead dislodgment.
Many ICDs deliver a programmed set of therapies per dysrhythmic episode. The number of therapies per episode is programming specific. If a delivered therapy does not terminate the arrhythmia, the device proceeds to the next programmed therapy. For example, a total of 6 attempts at defibrillation are attempted per episode of ventricular fibrillation. The device attempts defibrillation and then reevaluates the cardiac rhythm. If the arrhythmia persists, it delivers therapy number 2 and so on until all 6 attempts have been delivered. Once this occurs, the device does not deliver therapy until a new episode is declared. Initial therapy for ventricular tachycardia (VT) may be antitachycardia pacing (also known as overdrive pacing) rather than cardioversion.
ICDs do not prevent all sudden deaths, and acknowledging that cardiac arrest is not necessarily an ICD malfunction is important. The device may have properly delivered the required shocks for the triggering rhythm but still have been ineffective in resolving it.
Sprint Fidelis lead fractureIn July 2007, a higher than expected rate of Sprint Fidelis model 6949 ICD lead fractures were reported. Six patients presented with lead failure 4-23 months after implant. A subsequent database search for similar reports revealed that 33% of affected patients had inappropriate ICD shocks. Analysis of affected leads revealed 33% with high lead impedance and a 35% rate of pace-sense and high-voltage conductor fracture.
The lead manufacturer, Medtronic, Inc, issued an advisory in October 2007 with suggested ICD programming changes aimed at early detection of lead failure and reduction of inappropriate ICD shocks. Medtronic also discontinued sales of the affected leads, which include Sprint Fidelis models 6930, 6931, 6948, and 6949.[54]
A subsequent report found a 3.3% rate (17 of 514) of Sprint Fidelis lead failure 11-35 months after implant. Of the failures in this report, 88% were caused by pace-sense conductor fractures and 12% by high-voltage conductor defects. Of patients with pace-sense conductor fractures, 80% received inappropriate shocks. Notably, impedance monitoring did not prevent inappropriate shocks in two thirds of patients with lead failure.[55]
The Canadian Heart Rhythm Society issued a report on outcomes of the Medtronic Sprint Fidelis family of leads.[56] Lead failure was seen in 1.29% (80 of 6181) of patients at 21 months of observation. Inappropriate shocks were experienced in 56% of patients with lead failure. No deaths were attributed to lead failure. ICD interrogation prior to lead failure revealed evidence of altered lead function in only 10% of failing leads, consistent with the findings of Kallinen et al.[55]
The advisory issued by Medtronic suggested that, in general, the risks of lead replacement surgery outweigh the benefits.[54] Nonetheless, many centers are replacing leads for patients who are pacemaker dependent or who have received prior appropriate ICD shock therapy for treatment of appropriately detected, malignant ventricular arrhythmias.[56]
The Medtronic Sprint Fidelis leads (models 6949, 6948, 6931 and 6930) are subject to an increasing and problematic rate of lead failure.[57] . Specific device programming can enhance lead diagnosis, but many lead failures and the consequences of those failures remain unpredicted.[58]
Diagnostic information tracked and recorded in most devices may be used to distinguish between lead fracture and lead connection problems.[59] Current tools and technologies can be used to address Fidelis lead fractures, including a high success rate for lead extraction in specific centers; however, the optimal treatment strategy is evolving.[60]
A study by Morrison et al that sought to compare all-cause mortality in patients with Fidelis and Quattro leads with those with a nonadvisory lead found that of 2671 study patients, adjusted survival was similar with the Fidelis and Quattro leads.[61]
Implantation risk evaluationThe indication for ICD implantation represents a balance between potential benefit and likely risk. The acute risk of ICD implantation is small but is increased by multiple factors. The following are risk factors established by an ICD registry risk score model:[62]
Age greater than 70 years - 1 pointFemale - 2 pointsNYHA class III - 1 pointNYHA class IV - 3 pointsAtrial fibrillation - 1 pointPrior valve surgery - 3 pointsChronic lung disease - 2 pointsBlood urea nitrogen (BUN) > 30 mg/dL - 2 pointsReimplantation for reasons other than battery change - 6 pointsDual chamber ICD type - 2 pointsBiventricular ICD type - 4 pointsNonelective ICD implant -3 pointsThe risk of any inhospital complication increases from 0.6% among patients with a score of less than 5 to 8.4% among the patients with greater than 19 risk points.
The American College of Cardiology (ACC) and American Heart Association (AHA), in collaboration with the American Association for Thoracic Surgery and the Society of Thoracic Surgeons, have developed an extensive set of guidelines for ICD implantation. These guidelines represent a consensus statement that is largely evidence-based and that summarizes the available clinical evidence as of the time of its publication in May 2008.[2]
PreviousNextPacemaker ComplicationsPacemaker complications include malfunction due to mechanical factors such as pneumothorax, pericarditis, infection, skin erosion, hematoma, lead dislodgment, and venous thrombosis. Treatment depends on the etiology. Pneumothoraces may require medical observation, needle aspiration, or even chest tube placement.
Erosion of the pacer through the skin, while rare, requires device replacement and systemic antibiotics. Hematomas may be treated with direct pressure and observation, rarely requiring surgical drainage.
Lead dislodgment generally occurs within 2 days of device implantation pacer and may be seen on chest radiography. Alternatively, fluctuating impedance may be a subtle clue, as the patient may have normal impedance when the lead is in contact with the endocardium, but infinite (or very high) impedance when the lead is dislodged.
Free-floating ventricular leads may trigger malignant arrhythmias. Device-associated venous thrombosis is rare but generally presents as unilateral arm edema. Treatment includes extremity elevation and anticoagulation.
Advanced life support protocols, including defibrillation, may safely be performed for patients with pacemakers in place. Sternal paddles are placed at a safe distance (10cm) from the pulse generator. Temporary pacing may become necessary in cases of myocardial infarction, as the current pacemaker discharge settings may be insufficient to stimulate ventricular contraction.
PreviousNextPacemaker MalfunctionsMajor pacemaker malfunctions include the following:
Failure to outputFailure to captureFailure to sensePacemaker-mediated tachycardiaRunaway pacemakerPacemaker syndromeTwiddler's syndromeCardiac monitor pseudomalfunctionPacemaker pseudomalfunctionFailure to outputFailure to output occurs when no pacing artifact is present despite an indication to pace. This may be due to battery failure, lead fracture, fractured lead insulation, oversensing (inhibiting pacer output), poor lead connection at the takeoff from the pacer, and "cross-talk" (ie, a phenomenon occurring when atrial output is sensed by a ventricular lead in a dual-chamber pacer).
Management of pacer output complications includes medications to increase the intrinsic heart rate and placement of a temporary pacer. A chest radiograph is warranted to check pacer leads and to evaluate for possible lead fracture, which occurs most commonly at the clavicle or first rib. The patient's pacer identification card should be obtained and his or her electrophysiologist or cardiologist consulted.
Lead impedance (resistance) may also be an indicator of lead malfunction. Very low impedance may signify a fracture of the insulation (ie, the energy is dissipating into the surrounding tissue), whereas infinite (or very high) impedance may signify either complete lead fracture or a lead tip dislodged from the endocardium.
Failure to captureFailure to capture occurs when a pacing artifact is not followed by an atrial or a ventricular complex (see the image below). This may be due to the following:
Lead fractureLead dislodgementFractured lead insulationElevated pacing thresholdMI at the lead tipDrugs - Eg, flecainideMetabolic abnormalities - Eg, hyperkalemia, acidosis, alkalosisCardiac perforationPoor lead connection at the takeoff from the generatorImproper amplitude or pulse-width settings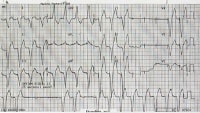 Intermittent periods of ventricular capture.
Intermittent periods of ventricular capture. Fibrosis at the endocardial surface where leads were implanted may also occur in the weeks following pacemaker implantation. The fibrosis may create an electrical resistance barrier preventing ventricular depolarization. This may detected as an abnormally high change in impedance (Δ impedance).
Managing pacer-capture complications is similar to treating output complications, with extra consideration given to treating metabolic abnormalities and potential MI. Temporary pacing is used to stabilize the patient until an electrophysiology technician or cardiologist can further evaluate the pacemaker.
OversensingOversensing occurs when a pacer incorrectly senses noncardiac electrical activity and is inhibited from pacing. This may result in a heart rate lower than the preset rate. This form of output failure may be due to muscular activity (particularly of the diaphragm or pectoralis muscles), EMI (from magnetic resonance imaging [MRI]), or fractured lead insulation. Oversensing is one condition that is diagnosable and treatable with magnet application. As mentioned before, magnet application will convert the pacemaker to asynchronous mode, and it will then operate at the preset rate.
Of note, it has been reported that cellular phones held within 10cm of the pulse generator may elicit this response.[63]
Individual ICD manufacturers also have recommendations for unsafe devices that may interact with the ICD (eg, Medtronic’s “Electromagnetic Compatibility Guideâ€[64] ).
UndersensingUndersensing occurs when a pacer incorrectly misses intrinsic depolarization and paces despite intrinsic activity. The pacemaker is more or less operating in asynchronous mode. This may be due to poor lead positioning, lead dislodgment, magnet application, low battery, or MI. Management is similar to that for other types of failures.
Pacemaker-mediated tachycardiaA premature ventricular contraction (PVC) in a dual-chamber pacemaker may precipitate a pacemaker-mediated tachycardia. If a PVC is transmitted in a retrograde manner through the atrioventricular node, it may, in turn, depolarize the atria. This atrial depolarization is detected by the atrial sensor, which then stimulates the ventricular leads to fire, hence creating an endless loop.
Although the maximum rate is limited by the pacemaker’s programmed upper limit, the possibility of developing ischemia exists in susceptible patients. This is another opportunity to use a magnet to diagnose and treat the arrhythmia. The magnet will place the pacemaker into asynchronous mode and sensing will be deactivated, thus preventing continuation of the reentrant dysrhythmia.
Runaway pacemakerA malfunction of the pacemaker generator resulting in a life-threatening rapid tachycardia (up to 200 beats per minute [bpm]) is known as runaway pacemaker. The generator may malfunction from various causes, although most commonly it is a battery failure or external damage.
This rare medical emergency requires immediate action. An external magnet may induce slower pacing, but it is possible that the device will not respond to magnet application and more aggressive measures may be necessary. If a patient becomes unstable, treatment involves making an incision in the chest wall over the pacemaker and severing the pacemaker leads from the generator. Note that the patient may require temporary pacing as a result.
Pacemaker syndromePacemaker syndrome is a phenomenon in which a patient feels symptomatically worse after pacemaker placement and presents with progressively worsening symptoms of congestive heart failure (CHF). This is mainly due to the loss of atrioventricular synchrony whereby the pathway is reversed and now has a ventricular origin. The atrial contribution to the preload is lost and cardiac output, as well as blood pressure, falls.
Immediate treatment is mainly supportive, whereas long-term treatment involves altering the pacemaker to restore atrioventricular synchrony and possible ventricular synchrony. For example, this may require changing the pacemaker from single-chamber to dual-chamber pacing or to dual-ventricular pacing.
Twiddler's syndromeSome patients will persistently disturb and manipulate the pacemaker generator, resulting in malfunction. A chest radiograph may reveal twisting or coiling or may show lead fracture, dislodgement, or migration. This situation will require surgical correction, with further patient education and counseling.
Cardiac monitor pseudomalfunctionFrom time to time, cardiac monitors will report an incorrect heart rate, too low or too high, due to inappropriate interpretation of pacing artifacts. Clinicians faced with this issue should first palpate the pulse and correlate with a pulse oximeter plethysmogram to verify the findings on a cardiac monitor. New monitors have settings to adapt for patients with pacemakers and provide more accurate heart rates.
Pacemaker pseudomalfunctionIn some clinical settings, an apparent pacing system malfunction is suggested; however, the apparent malfunction is a normal, programmed pacer function. Such pseudomalfunctions are partly due to new algorithms to preserve intrinsic conduction and more physiologic pacing. These can sometimes be corrected by changing the programming; in other cases, the patient may need to have the device changed.
Magnet use inhibits further ICD discharge. It does not, however, inhibit pacing. In some devices, application of a magnet produces a soft beep for each QRS complex. If the magnet is left on for approximately 30 seconds, the ICD is disabled and a continuous tone is generated. To reactivate the device, the magnet must be lifted off the area of the generator and then replaced. After 30 seconds, the beep returns for every QRS complex. Indications for ICD deactivation are as follows:
End-of-life care - After a discussion with the patient and familyInappropriate shocksDuring resuscitationWith transcutaneous pacing - External pacing can cause an ICD to fireDuring procedures such as central lines or surgery with electrocauteryPreviousNextInpatient CareOne of the most difficult decisions after a patient presents to the ED complaining of an implantable cardioverter-defibrillator (ICD) discharge is to determine if the discharge was appropriate. Whenever possible, the device should be interrogated, since, unless the shock and the rhythm that preceded it were witnessed, it is not possible to determine shock appropriateness without investigation.
Reasons for admission may include the following:
Device investigation - To determine whether there is an imminent battery failure (multiple shocks will deplete battery life)Addition of antiarrhythmic medicationsTreatment of MI (which may be linked to the initial discharge)Treatment of patient discomfortProvision of psychological support - Up to 35% of patients develop anxiety disorder following ICD placement[3] PreviousNextResuscitation of Patients With an ICDIf a patient enters a life-threatening cardiac arrhythmia, advanced cardiac life support (ACLS) protocols should be initiated immediately. Although an implantable cardioverter-defibrillator (ICD) will attempt defibrillation, chest compressions should be continued. Note that some of the current may enter the rescuer; aside from some mild discomfort, however, there has never been a reported case of rescuer injury from this.[45]
VT and VF refractory to ICD defibrillation will require external defibrillation and/or antiarrhythmic medications as dictated by ACLS protocols. If external defibrillation is required, attempt to keep the generator at least 10cm away and out of the shock wave. Defibrillation that affects the generator may cause total device failure. However, do not withhold therapy for fear of damaging the ICD.
If rescuers are uncomfortable with ICD discharge during resuscitation, deactivation of the ICD with a magnet is indicated.
PreviousNextCentral Venous Catheter Placement in ICD PatientsPacemaker or implantable cardioverter-defibrillator (ICD) leads placed in the venous system often have surrounding thrombosis, with 20% of patients having complete occlusion at 2 years.[65] If a metal guidewire contacts the lead system during central line placement, there may be enough noisy artifact to trigger an inappropriate shock.
Consideration should be given to either avoid a metal guidewire or deactivate the ICD during central line placement. Although the contralateral subclavian or internal jugular vein can be cannulated with care, femoral vein access is a much safer option.
PreviousNextConsultations and MonitoringSome patients with implantable cardioverter-defibrillator (ICDs) require emotional or psychological support for anxiety, depression, and difficulties in adjusting to life with an ICD. The dedicated ICD clinic staff can help with many of these issues. Referral to a psychologist or psychiatrist may also be helpful. Support groups are available for patients and their families, some of which are accessible online.
Patients with ICDs should be observed in a dedicated ICD clinic. Patients are seen more frequently early after implant—generally, 1 week after implant for a wound check, 1 month after implant for device interrogation, and 3 months after implant for repeat device interrogation. The follow-up interval generally can be increased to every 6 months in patients who are clinically stable.
PreviousNextPatient EducationAlthough technologic advances have greatly reduced the potential effects of EMI, patients should be advised to avoid strong electromagnetic fields because of potential interference with sensing circuitry. Examples of potential hazards include arc welders, large generators, and magnetic resonance imaging (MRI) magnets. Household appliances, microwave ovens, cell phones, and hand-held metal detectors (used for security screening) should not pose a serious threat.[66]
A shock from an implantable cardioverter-defibrillator (ICD) is generally painful. Patients should be advised of this in advance. Advise patients and their families that someone touching them is not harmed if the ICD discharges. Issues regarding driving can be problematic. In the absence of specific state laws, many electrophysiologists recommend that patients be shock-free for 6 months before resuming driving. Loss of driving privileges imposes an enormous burden and change of lifestyle on patients with this restriction. Rules and recommendations regarding commercial driving typically are more stringent.
Previous Contributor Information and DisclosuresAuthorDaniel M Beyerbach, MD, PhD Medical Director, Cardiac Rhythm Program, The Christ Hospital; Affiliate Clinical Assistant Professor of Biomedical Science, Florida Atlantic University
Daniel M Beyerbach, MD, PhD is a member of the following medical societies: American College of Cardiology
Disclosure: Nothing to disclose.
Jeffrey N Rottman, MD Professor of Medicine and Pharmacology, Vanderbilt, Pacemakers and Implantable Cardioverter-Defibrillators






0 comments:
Post a Comment