Renovascular hypertension (RVHT) reflects the causal relation between anatomically evident arterial occlusive disease and elevated blood pressure. The coexistence of renal arterial vascular (ie, renovascular) disease and hypertension roughly defines this type of nonessential hypertension. More specific diagnoses are made retrospectively when hypertension is improved after intravascular intervention.[1]
Since Goldblatt’s seminal experiment in 1934, RVHT has increasingly been recognized as an important cause of clinically atypical hypertension and chronic kidney disease, the latter by virtue of renal ischemia. RVHT is the clinical consequence of activation of the renin-angiotensin-aldosterone system (RAAS).
Renal artery occlusion creates ischemia, which triggers the release of renin and a secondary elevation in blood pressure. Hyperreninemia promotes conversion of angiotensin I to angiotensin II, causing severe vasoconstriction and aldosterone release. The ensuing cascade of events varies, depending on the presence of a functioning contralateral kidney.
When 2 kidneys are present, aldosterone-mediated sodium and water retention is handled properly by the nonstenotic kidney, precluding volume from contributing to the angiotensin II–mediated hypertension. By contrast, a solitary ischemic kidney has little or no capacity for sodium and water excretion; hence, volume plays an additive role in the hypertension.
At present, no sufficiently accurate, noninvasive, radiologic, or serologic screening test is available that, if negative, completely excludes the presence of renal artery stenosis (RAS). Current guidelines of the American College of Cardiology (ACC) and the American Heart Association (AHA) advocate screening for RAS only when a corrective procedure will be considered if renovascular disease is detected.
When the history is highly suggestive and no risk of radiocontrast-mediated renal injury is present, intra-arterial digital subtraction angiography (DSA) or conventional angiography (renal arteriography) is the appropriate initial test. When a moderate suspicion of renovascular disease exists, spiral computed tomography (CT), magnetic resonance angiography (MRA), or duplex ultrasonography should be performed, depending on availability and local experience.
Antihypertensive drug therapy is indicated. Optimal blood pressure control plays an essential role in the therapeutic management of RVHT; however, aggressive control of other risk factors for atherosclerosis also is crucial. Cessation of smoking is important for its positive impact on the cardiovascular risk profile in patients with hypertension. Similarly, antidyslipidemic therapy for those patients with hyperlipidemia likely provides benefit in atherosclerotic RVHT.
The invasive and surgical options for treatment of RVHT include percutaneous transluminal angioplasty (PTA), surgical revascularization, and nephrectomy.
Patient education regarding hypertension should include information about the clinical features associated with RVHT (see Presentation) and about the importance of good blood pressure control.
NextPathophysiologyThe chief pathophysiologic mechanism underlying RVHT involves activation of both limbs of the RAAS and depends on the presence or absence of a contralateral kidney (see the image below).
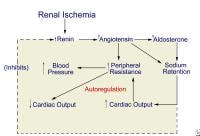 Proposed pathogenesis of renovascular hypertension.
Proposed pathogenesis of renovascular hypertension. Unilateral renal ischemia initiates hypersecretion of renin, which accelerates conversion of angiotensin I to angiotensin II and enhances adrenal release of aldosterone. The result is profound angiotensin-mediated vasoconstriction and aldosterone-induced sodium and water retention.
In the 2-kidney 1-clip model, where the clinical correlate is unilateral renal artery disease, sodium and water handling via pressure diuresis of the contralateral kidney may be sufficient to prevent a volume component to the hypertension. In the setting of a solitary kidney (experimentally, the 1-kidney 1-clip model), sodium and water handling is compromised, sodium and water retention ensues, and volume-mediated hypertension occurs.
In unilateral RAS, renin production is increased in the ischemic kidney but suppressed in the unaffected nonstenotic kidney, which lacks the same ischemic stimulus. Consequently, when 2 kidneys are present with a unilateral stenosis (2-kidney 1-clip model), hyperreninemia persists and blood pressure remains elevated because of an angiotensin II–induced vasoconstrictive effect. Renin production decreases in the contralateral kidney, a pressure diuresis ensues, and hypertension is maintained by high levels of angiotensin II.
A solitary kidney rendered ischemic by RAS is unable to achieve the pressure diuresis required to handle the aldosterone-induced sodium and water retention. The resultant volume expansion contributes to the elevation in blood pressure and also suppresses the production of renin by the stenotic kidney.
The sympathetic nervous system does not appear to play a role in perpetuating elevated blood pressure in the 2-kidney 1-clip model of RVHT. Evidence for a role in the 1-kidney 1-clip model of RVHT has been presented but is not clear or definitive.
Stages in development of renovascular hypertensionThe evolution of RVHT has been described as having the following 3 stages or phases:
Renin-angiotensin–dependent phaseSalt-retention phaseSystemic renin-angiotensin–independent phaseIn the first phase, the immediate rise in blood pressure is a direct consequence of hyperreninemia. Over days to weeks, blood pressure remains elevated, but the course and presence of hyperreninemia vary with the presence and function of the contralateral kidney. The mechanism by which hypertension is produced in patients with renovascular disease thus changes over time and varies with the state of sodium balance.
When the contralateral kidney is functional, volume expansion is avoided and renin levels remain high. The 2 kidneys are in opposition; the stenotic kidney avidly retains sodium and produces excess renin in response to renal ischemia, while the nonstenotic kidney excretes sodium and water to maintain euvolemia and renin production decreases. The end result is systemic hypertension that is mediated by both renin and angiotensin.
In the second phase, in the setting of an ischemic solitary kidney, sodium and water retention, together with the vasopressor effects of angiotensin II, act to maintain renal perfusion pressure. The stimulus to produce renin is stifled, and renin levels fall. Hypertension becomes less dependent on angiotensin II and predominantly results from volume expansion. Thus, perfusion pressure is restored at the expense of systemic hypertension and volume overload.
If blood flow is restored during these first 2 phases and renal perfusion is reinstated, blood pressure soon returns to a normal level. If renal hypoperfusion persists and the third phase is reached, restoration of renal blood flow may not normalize blood pressure, presumably because of secondary irreversible vascular or renal parenchymal disease.
In the third phase, hypertension often is unremitting, persisting well after the removal of the stenosis. Recalcitrant hypertension in this setting likely represents the presence of ischemic nephropathy in either or both kidneys; patients in whom stenoses were not hemodynamically significant initially also may have persistent hypertension.
RAAS and control of intrarenal hemodynamicsAngiotensin II exerts a vasoconstrictive effect on both afferent and efferent arterioles, but because the efferent arteriole has a smaller basal diameter, the increase in efferent resistance exceeds that the increase in afferent resistance. Afferent vasoconstriction is further minimized by angiotensin II–mediated release of vasodilatory prostaglandins and nitric oxide. In addition, angiotensin II can constrict the glomerular mesangium, thereby reducing the surface area available for filtration.
The net effect of angiotensin II on filtration invokes the opposing factors of reduced renal blood flow and mesangial surface area (causing a decrease in filtration) and the increase in glomerular capillary pressure (which tends to increase filtration). The end result depends on the clinical setting in which it occurs.
In the healthy kidney, a fall in systemic blood pressure activates the RAAS, which triggers a decrease in renal blood flow secondary to increased renal vascular (afferent) resistance. The preferential increase in efferent resistance mediated by angiotensin II results in increased glomerular capillary hydraulic pressure, which maintains the glomerular filtration rate (GFR).
In the ischemic kidney with reduced afferent blood flow, intraglomerular pressure and glomerular filtration are maintained by and depend upon angiotensin II–mediated efferent vasoconstriction. In this setting, removal of the efferent vasoconstrictive effect by angiotensin blockade, as achieved by angiotensin-converting enzyme (ACE) inhibitors, results in a decrease in intraglomerular pressure and GFR.
Thus, in patients with renovascular disease, particularly those with bilateral RAS or those with a stenotic renal artery to a single kidney, ACE inhibitors may cause a deterioration of renal function and azotemia. The propensity for angiotensin receptor blockers (ARBs) to affect GFR adversely is based on similar pathophysiology. It should be kept in mind that an acute decline in renal function in this setting is reversible once the ACE inhibitor (or the ARB) is discontinued.
ManifestationsIn adults, renovascular disease tends to appear at different times and affects the sexes differently. Atherosclerotic disease affects mainly the proximal third of the main renal artery and is most common among older men. Fibromuscular dysplasia (FMD) involves the distal two thirds and branches of the renal arteries and is most common among younger women. Midaortic syndrome is considered a variant of FMD. Neurofibromatosis may be seen.
Fibromuscular dysplasia
FMD involves fibrous or muscular hypertrophy of the vessel tunica media with fibrous intimal hyperplasia; accordingly, it is sometimes referred to as fibromuscular hyperplasia. Often, poststenotic dilatation is also present. The process may range from mild occlusion to complete occlusion of the vessel. On radiographs, FMD produces the classic string-of-beads appearance less often in children than in adults; rather, it tends to show short discrete or longer tubular segments of stenosis.
The most common site of stenosis is the orifice of the renal artery at its origin in the aortic wall (see the images below). The next most common location is within the main renal artery, and the segmental arteries are the least common site of stenosis. Total occlusion most often occurs at the orifice of the renal artery.
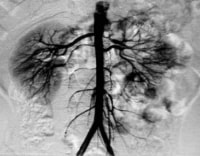 Aortogram of 4-year-old child with renovascular hypertension caused by stenosis of left renal artery. Note that left kidney has 2 renal arteries and that artery to superior pole has stenosis.
Aortogram of 4-year-old child with renovascular hypertension caused by stenosis of left renal artery. Note that left kidney has 2 renal arteries and that artery to superior pole has stenosis. 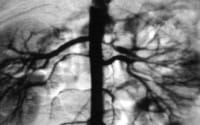 Close-up view of aortogram of 4-year-old child. Stenotic lesion begins at ostium of left superior renal artery. This lesion was caused by fibromuscular dysplasia and did not respond well to balloon angioplasty.
Close-up view of aortogram of 4-year-old child. Stenotic lesion begins at ostium of left superior renal artery. This lesion was caused by fibromuscular dysplasia and did not respond well to balloon angioplasty. FMD may be either unilateral or bilateral, but the inciting event is unknown. Some have suggested an autoimmune origin. In 1995, Stanley proposed that the lesion forms as a developmental disease in the muscular layer, which is followed by intimal hyperplasia from the abnormal flow through the constricted lumen.[2]
Midaortic syndrome
In midaortic syndrome, vascular involvement extends beyond the renal artery. Aortic narrowing is present, often extending from the aortic hiatus to just above the inferior mesenteric artery (IMA). One or both of the renal arteries are usually involved, and the celiac artery and the superior mesenteric artery (SMA) may be narrowed. Midaortic syndrome may result in total renal artery occlusion, with perfusion dependent on collateral circulation. Extensive collateralization from the IMA and a Riolan arcade may exist. Renal artery stenosis is usually bilateral.
Neurofibromatosis
Hypertension in patients with neurofibromatosis is often essential, but some patients also present with RVHT (see the image below). These patients have a pattern of RAS similar to that observed in FMD. However, involvement of the intrarenal arteries and arterioles may also exist. Neurofibromatosis usually involves the renal arteries of both kidneys.
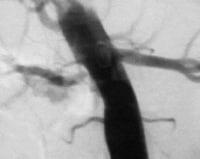 Aortogram of 8-year-old child with neurofibromatosis and renovascular hypertension caused by right renal artery stenosis. PreviousNextEtiology
Aortogram of 8-year-old child with neurofibromatosis and renovascular hypertension caused by right renal artery stenosis. PreviousNextEtiologyThe term renovascular hypertension implies that the cause of the elevated blood pressure is decreased arterial inflow to the kidneys. Overall, approximately two thirds of RVHT cases are caused by atherosclerotic disease, and one third are caused by FMD or other congenital disorders. Other clinical entities that may be associated with RVHT include cholesterol embolic disease, acute arterial thrombosis or embolism, aortic dissection, renal arterial trauma, arterial aneurysm, arteriovenous malformation of the renal artery, and polyarteritis nodosa.
In children, congenital disorders that may lead to RVHT include arterial hypoplasia (as observed in multicystic renal dysplasia), neurofibromatosis, and Williams syndrome. Tumors and other masses may impinge on the renal vasculature. Trauma, irradiation, vessel anastomosis in transplantation, and thrombosis all may lead to constriction of vessels and the resulting hypertension. The most common cause of RVHT in newborns is a thrombosis or embolization related to umbilical artery catheterization.
More commonly, RVHT in children is considered an acquired disease. FMD is the most common form of acquired RVHT. Its incidence varies geographically, but in the United States, it is the most common cause of secondary hypertension in children. Other forms of acquired RVHT include subisthmic coarctation, Moyamoya disease, Takayasu arteritis, Kawasaki disease, vasculitis, vascular trauma, renal artery thrombosis, tumors, midaortic syndrome, or anastomotic stenosis (eg, after transplantation).
Trauma or kidney transplantation can lead to scarring or anastomotic lesions that produce renovascular constriction. Although Takayasu arteritis and Kawasaki disease occasionally lead to FMD, the cause of FMD is not always known. Radiation therapy of tumors in the renal area may lead to renovascular hypertension.
Prenatal detection of multicystic renal dysplasia by means of screening ultrasonography is common. These lesions are rarely bilateral and are usually associated with ipsilateral ureteral atresia. Hypertension and recurrent infections can result from this condition.
PreviousNextEpidemiologyUnited States statisticsRVHT is the most common type of secondary hypertension, accounting for 1-5% of cases in unselected populations and as many as 30% of cases in selected populations. The prevalence may be up to 60% in patients older than 70 years.
The incidence of hypertension in children is reported to be 1-5%, and that in adolescents may be as high as 10%. In children, unlike adults, 70-80% of cases of hypertension may be due to secondary hypertension, which is often correctable. Of those children with secondary hypertension, 5-25% have elevated blood pressure with a renovascular etiology.
RVHT is common in children and is second only to coarctation of the aorta as a surgically correctable cause of hypertension. With improved screening for coarctation in younger children, RVHT may become the most common cause of surgically correctable hypertension.
International statisticsThe prevalence of RVHT internationally is not clear, but it likely accounts for the sole etiology in a relatively small percentage of unselected patients with hypertension. Significant geographic differences in the overall prevalence of RVHT have not been reported, though the etiology does appear to vary geographically.
In the western hemisphere, FMD is the most common cause of pediatric renovascular hypertension. Reports from Asia identify arteritis, either aortoarteritis or Takayasu arteritis, as the most common cause of renovascular hypertension in children. One study in south Asia found that 87% of the patients presenting with renovascular hypertension had arteritis.[3] A report from South Africa also indicated that Takayasu arteritis was the most important cause of renovascular hypertension in nonwhite children.
Age-related demographicsThe onset of RVHT tends to occur in patients younger than 30 years or older than 50 years. Systemic hypertension is less common in children than in adults, but the incidence of hypertension in children is approximately 1-5%. The presence of hypertension in younger children is usually indicative of an underlying disease process (secondary hypertension). In children, approximately 5-25% of secondary hypertension is attributed to renovascular disease.
In children, the prevalence of renovascular disease as the cause of hypertension is inversely related to age. In other words, younger children are more likely to have hypertension that is due to renovascular disease. In children younger than 5 years, the incidence of potentially surgically correctable hypertension is close to 80%. This incidence drops to 40-45% in children aged 6-10 years. In children aged 11-20 years, a 20% incidence of surgically correctable hypertension is observed.
Sex-related demographicsRVHT is most common in younger women and older men. Younger women develop RVHT most commonly from FMD affecting the distal two thirds and branches of the renal arteries. Older men develop RVHT most often from atherosclerotic disease affecting mainly the proximal third of the main renal artery. In children, multiple studies have failed to demonstrate any clear sex difference with regard to the prevalence of RVHT.
Race-related demographicsOverall, RVHT and RAS are less common in the black population than in the white population. Blood pressure has been shown to be higher in black children than in white children, but the difference has not been deemed clinically significant. When adjusted for height, much of this difference is eliminated. RVHT is less common among older black children than among adolescent whites, but the prevalence is actually higher in young black children.
PreviousNextPrognosisThe prognosis of patients with RVHT is difficult to ascertain and varies with the extent of the occlusive phenomena, the sensitivity of the individual to antihypertensive therapy, and the efficacy of surgical repair or angioplasty. In patients with hypertension, the presence of atherosclerotic renal artery disease is a strong predictor of increased mortality relative to the general population. RVHT in the setting of renal dysfunction is associated with the greatest mortality.
Although the actual mortality of untreated renovascular hypertension has not been reported, in part because effective treatments are available, the prognosis is clearly poor. The severity of the hypertension places considerable of strain on target organs and can lead to death. Fortunately, most renovascular disease is correctable with surgical treatment or invasive intervention.
For 35% of patients, PTA yields normal blood pressures. Another one third of patients have decreased blood pressures. Unfortunately, a high rate of recurrence of hypertension and vascular stenosis appears to be observed in patients treated with PTA.
Surgical revascularization provides a very good prognosis for patients with renovascular hypertension. Approximately 70% of patients become normotensive without requiring additional pharmacologic treatment. Another 25% have reduced hypertension that can usually be resolved with the addition of medical therapy. Thus, fewer than 5% of patients appear refractory to revascularization. Some patients may experience resolution of their hypertension after nephrectomy.
Successful surgical intervention is expected to offer patients a normal lifespan without complications. Children who undergo surgical revascularization appear to do well for at least 16 years postoperatively. They are able to participate in active sports and similar vigorous activities without problems. Further long-term follow-up is needed to determine the durability of these reconstructions and the actual life potential of these children.
PreviousProceed to Clinical Presentation , Renovascular Hypertension





0 comments:
Post a Comment