Aortic stenosis is the obstruction of blood flow across the aortic valve. Among symptomatic patients with medically treated moderate-to-severe aortic stenosis, mortality from the onset of symptoms is approximately 25% at 1 year and 50% at 2 years. Symptoms of aortic stenosis usually develop gradually after an asymptomatic latent period of 10-20 years.
Essential update: Expanded FDA labeling for transcatheter valve allows alternative access sitesIn September 2013, the FDA approved new labeling for the Sapien transcatheter valve, which eliminates references to specific access sites used when implanting the valves (ie, transfemoral and transapical approaches) and allows the use of alternative access sites (eg, subclavian approach).[1, 2] The change in labeling was supported by data from the Transcatheter Valve Therapy Registry and European registries.
Signs and symptomsThe classic triad of symptoms in patients with aortic stenosis is as follows[3] :
Chest pain: Angina pectoris in patients with aortic stenosis is typically precipitated by exertion and relieved by restHeart failure: Symptoms include paroxysmal nocturnal dyspnea, orthopnea, dyspnea on exertion, and shortness of breathSyncope: Often occurs upon exertion when systemic vasodilatation in the presence of a fixed forward stroke volume causes the arterial systolic blood pressure to declineSystolic hypertension can coexist with aortic stenosis. However, a systolic blood pressure higher than 200 mm Hg is rare in patients with critical aortic stenosis.
In severe aortic stenosis, the carotid arterial pulse typically has a delayed and plateaued peak, decreased amplitude, and gradual downslope (pulsus parvus et tardus).
Other symptoms of aortic stenosis include the following:
Pulsus alternans: Can occur in the presence of left ventricular systolic dysfunctionHyperdynamic left ventricle: Unusual; suggests concomitant aortic regurgitation or mitral regurgitationSoft or normal S1Diminished or absent A2: The presence of a normal or accentuated A2 speaks against the existence of severe aortic stenosisParadoxical splitting of the S2: Resulting from late closure of A2Accentuated P2: In the presence of secondary pulmonary hypertensionEjection click: Common in children and young adults with congenital aortic stenosisProminent S4: Resulting from forceful atrial contraction into a hypertrophied left ventricleSystolic murmur: The classic crescendo-decrescendo systolic murmur of aortic stenosis begins shortly after the first heart sound; the intensity increases toward midsystole and then decreases, with the murmur ending just before the second heart soundSee Clinical Presentation for more detail.
DiagnosisThe following studies are used in the diagnosis and assessment of aortic stenosis:
Serum electrolyte levelsCardiac biomarkersComplete blood countB-type natriuretic peptide: May provide incremental prognostic information for predicting symptom onset in asymptomatic patients with severe aortic stenosis[4] Electrocardiography: Serial ECG can demonstrate the progression of aortic stenosisChest radiographyEchocardiography: 2-dimensional and DopplerCardiac catheterization: Can be used if clinical findings are inconsistent with echocardiogram resultsCoronary angiographyRadionuclide ventriculography: May provide information on LV functionExercise stress testing: Contraindicated in symptomatic patients with severe aortic stenosisSee Workup for more detail.
ManagementThe only definitive treatment for aortic stenosis is aortic valve replacement. The development of symptoms due to this condition provides a clear indication for replacement.[5, 6]
Emergency care
A patient presenting with uncontrolled heart failure should be treated supportively with oxygen, cardiac and oximetry monitoring, intravenous access, loop diuretics, nitrates (remembering the potential nitrate sensitivity of patients with aortic stenosis), morphine (as needed and tolerated), and noninvasive or invasive ventilatory support (as indicated). Patients with severe heart failure due to aortic stenosis that is resistant to medical management should be considered for urgent surgery.
Pharmacologic therapy
Agents used in the treatment of patients with aortic stenosis include the following:
Digitalis, diuretics, and angiotensin-converting enzyme (ACE) inhibitors: Can be cautiously used in patients with pulmonary congestion Vasodilators: May be used for heart failure and for hypertension but should also be employed with extreme cautionDigoxin, diuretics, ACE inhibitors, or angiotensin receptor blockers[6] : Recommended by the European Society of Cardiology (ESC)/European Association for Cardio-Thoracic Surgery (EACTS) guidelines for patients with heart failure symptoms who are not suitable candidates for surgery or transcatheter aortic valve implantationAortic valve replacement
According to American College of Cardiology (ACC)/American Heart Association (AHA) guidelines, candidates for aortic valve replacement include the following patients[7] :
Symptomatic patients with severe aortic stenosisPatients with severe aortic stenosis undergoing coronary artery bypass surgeryPatients with severe aortic stenosis undergoing surgery on the aorta or other heart valvesPatients with severe aortic stenosis and LV systolic dysfunction (ejection fractionPercutaneous balloon valvuloplasty
Percutaneous balloon valvuloplasty is used as a palliative measure in critically ill adult patients who are not surgical candidates or as a bridge to aortic valve replacement in critically ill patients.
See Treatment and Medication for more detail.
Image library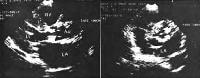 Calcific aortic stenosis (parasternal long-axis and short-axis views). NextBackground
Calcific aortic stenosis (parasternal long-axis and short-axis views). NextBackgroundAortic stenosis is the obstruction of blood flow across the aortic valve. Aortic stenosis has several etiologies, including congenital (unicuspid or bicuspid valve), calcific (due to degenerative changes), and rheumatic. Degenerative calcific aortic stenosis is now the leading indication for aortic valve replacement. The favorable long-term outcome following aortic valve surgery and the relatively low operative risk emphasize the importance of an accurate and timely diagnosis (see Prognosis).
Stenotic valves are shown in the images below. Symptoms of aortic stenosis usually develop gradually after an asymptomatic latent period of 10-20 years. Exertional dyspnea or fatigue is the most common initial complaint. Ultimately, most patients experience the classic triad of chest pain, heart failure, and syncope (see History).
Two-dimensional (2D) Doppler echocardiography is the imaging modality of choice to diagnose and estimate the severity of aortic stenosis and localize the level of obstruction (see Workup). The only definitive treatment for aortic stenosis is aortic valve replacement (see Treatment and Management).
Go to Pediatric Valvar Aortic Stenosis, Pediatric Subvalvar Aortic Stenosis, and Pediatric Supravalvar Aortic Stenosis for more complete information on these topics.
PreviousNextPathophysiologyWhen the aortic valve becomes stenotic, resistance to systolic ejection occurs and a systolic pressure gradient develops between the left ventricle and the aorta. This outflow obstruction leads to an increase in left ventricular (LV) systolic pressure. As a compensatory mechanism to normalize LV wall stress, LV wall thickness increases by parallel replication of sarcomeres, producing concentric hypertrophy. At this stage, the chamber is not dilated and ventricular function is preserved, although diastolic compliance is reduced.
Eventually, however, LV end-diastolic pressure (LVEDP) rises, which causes a corresponding increase in pulmonary capillary arterial pressures and a decrease in cardiac output due to diastolic dysfunction. The contractility of the myocardium may also diminish, which leads to a decrease in cardiac output due to systolic dysfunction. Ultimately, heart failure develops.
In most patients with aortic stenosis, LV systolic function is preserved and cardiac output is maintained for many years despite an elevated LV systolic pressure. Although cardiac output is normal at rest, it often fails to increase appropriately during exercise, which may result in exercise-induced symptoms.
Diastolic dysfunction may occur as a consequence of impaired LV relaxation and/or decreased LV compliance, as a result of increased afterload, LV hypertrophy, or myocardial ischemia. LV hypertrophy often regresses following relief of valvular (also called valvular) obstruction. However, some individuals develop extensive myocardial fibrosis, which may not resolve despite regression of hypertrophy.
In patients with severe aortic stenosis, atrial contraction plays a particularly important role in diastolic filling of the left ventricle. Thus, development of atrial fibrillation in aortic stenosis often leads to heart failure due to an inability to maintain cardiac output.
Increased LV mass, increased LV systolic pressure, and prolongation of the systolic ejection phase all elevate the myocardial oxygen requirement, especially in the subendocardial region. Although coronary blood flow may be normal when corrected for LV mass, coronary flow reserve is often reduced.
Myocardial perfusion is thus compromised by the relative decline in myocardial capillary density and by a reduced diastolic transmyocardial (coronary) perfusion gradient due to elevated LV diastolic pressure. Therefore, the subendocardium is susceptible to underperfusion, which results in myocardial ischemia.
Angina results from a concomitant increased oxygen requirement by the hypertrophic myocardium and diminished oxygen delivery secondary to diminished coronary flow reserve, decreased diastolic perfusion pressure, and relative subendocardial myocardial ischemia.
PreviousNextEtiologyMost cases of aortic stenosis are due to the obstruction at the valvular level. Common causes are summarized in Table 1.
Table 1. Common Causes of Aortic Stenosis Among Patients Requiring Surgery (Open Table in a new window)
Age Age >70 years (n=322) Bicuspid AV (50%)Postinflammatory (25%)
Degenerative (18%)
Unicommissural (3%)
Hypoplastic (2%)
Indeterminate (2%)
Degenerative (48%)
Bicuspid (27%)
Postinflammatory (23%)
Hypoplastic (2%)
Valvular aortic stenosis can be either congenital or acquired.
Congenital valvular aortic stenosisCongenitally unicuspid, bicuspid, tricuspid, or even quadricuspid valves may be the cause of aortic stenosis. In neonates and infants younger than 1 year, a unicuspid valve can produce severe obstruction and is the most common anomaly in infants with fatal valvular aortic stenosis. In patients younger than 15 years, unicuspid valves are most frequent in cases of symptomatic aortic stenosis.
In adults who develop symptoms from congenital aortic stenosis, the problem is usually a bicuspid valve. Bicuspid valves do not cause significant narrowing of the aortic orifice during childhood. The altered architecture of the bicuspid aortic valve induces turbulent flow with continuous trauma to the leaflets, ultimately resulting in fibrosis, increased rigidity and calcification of the leaflets, and narrowing of the aortic orifice in adulthood.
A cohort study by Tzemos et al of 642 ambulatory adults with bicuspid aortic valves found that during the mean follow-up duration of 9 years, survival rates were not lower than for the general population. However, young adults with bicuspid aortic valve had a high likelihood of eventually requiring aortic valve intervention.[8]
Congenitally malformed tricuspid aortic valves with unequally sized cusps and commissural fusion (“functionally bicuspid†valves) can also cause turbulent flow leading to fibrosis and, ultimately, to calcification and stenosis. Clinical manifestations of congenital aortic stenosis in adults usually appear after the fourth decade of life.
Acquired valvular aortic stenosisThe main causes of acquired aortic stenosis include degenerative calcification and, less commonly, rheumatic heart disease.
Degenerative calcific aortic stenosis (also called senile calcific aortic stenosis) involves progressive calcification of the leaflet bodies, resulting in limitation of the normal cusp opening during systole. This represents a consequence of long-standing hemodynamic stress on the valve and is currently the most frequent cause of aortic stenosis requiring aortic valve replacement. The calcification may also involve the mitral annulus or extend into the conduction system, resulting in atrioventricular or intraventricular conduction defects.
Risk factors for degenerative calcific aortic stenosis include hypertension, hypercholesterolemia, diabetes mellitus, and smoking. The available data suggest that the development and progression of the disease are due to an active disease process at the cellular and molecular level that shows many similarities with atherosclerosis, ranging from endothelial dysfunction to, ultimately, calcification.[9]
In rheumatic aortic stenosis, the underlying process includes progressive fibrosis of the valve leaflets with varying degrees of commissural fusion, often with retraction of the leaflet edges and, in certain cases, calcification. As a consequence, the rheumatic valve often is regurgitant and stenotic. Coexistent mitral valve disease is common.
Other, infrequent causes of aortic stenosis include obstructive vegetations, homozygous type II hypercholesterolemia, Paget disease, Fabry disease, ochronosis, and irradiation.
It is worthwhile to note that although differentiation between tricuspid and bicuspid aortic stenosis is frequently made, it is often difficult to determine the number of aortic valve leaflets. A study comparing operatively excised aortic valve structure evaluation by cardiac surgeon versus pathologist found that valve structure determination was frequently incongruous.[10]
PreviousNextEpidemiologySevere aortic stenosis is rare in infancy, occurring in 0.33% of live births, and is due to a unicuspid or bicuspid valve. Most patients with a congenitally bicuspid aortic valve who develop symptoms do not do so until middle age or later. Patients with rheumatic aortic stenosis typically present with symptoms after the sixth decade of life.
Aortic sclerosis (aortic valve calcification without obstruction to blood flow, considered a precursor of calcific degenerative calcific aortic stenosis) increases in incidence with age and is present in 29% of individuals older than 65 years and in 37% of individuals older than 75 years. In elderly persons, the prevalence of aortic stenosis is between 2% and 9%.
Degenerative calcific aortic stenosis usually manifests in individuals older than 75 years and occurs most frequently in males.[5]
PreviousNextPrognosisPatients with severe aortic stenosis may be asymptomatic for many years despite the presence of severe LV outflow tract obstruction (LVOTO). LVOTOs have been associated with “high heritability.†One study suggests that 20% of patients with isolated LVOTO had an affected first-degree relative with undetected bicuspid aortic valves.[11]
Asymptomatic patients, even with critical aortic stenosis, have an excellent prognosis for survival, with an expected death rate of less than 1% per year; only 4% of sudden cardiac deaths in severe aortic stenosis occur in asymptomatic patients. A new proposed aortic stenosis grading classification that integrates valve area and flow-gradient patterns has been found to allow for better characterization of the clinical outcome among patients with asymptomatic severe aortic stenosis.[12]
Although the presence of low-gradient "severe stenosis" (defined as aortic valve area 2 and mean gradient 40 mm Hg) is considered by some to be associated with a poor prognosis, the prospective Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) study found that such patients have an outcome similar to that of patients with moderate stenosis.[13]
Among symptomatic patients with medically treated, moderate-to-severe aortic stenosis, mortality rates from the onset of symptoms are approximately 25% at 1 year and 50% at 2 years. More than 50% of deaths are sudden. In patients in whom the aortic valve obstruction remains unrelieved, the onset of symptoms predicts a poor outcome with medical therapy; the approximate time interval from the onset of symptoms to death is 1.5-2 years for heart failure, 3 years for syncope, and 5 years for angina.
Although the obstruction tends to progress more rapidly in degenerative calcific aortic valve disease than in congenital or rheumatic disease, predicting the rate of progression in individual patients is not possible. Catheterization and echocardiographic studies suggest that, on average, the valve area declines 0.1-0.3 cm2 per year; the systolic pressure gradient across the valve can increase by as much as 10-15 mm Hg per year. Obstruction progresses more rapidly in elderly patients with coronary artery disease and chronic renal insufficiency.
PreviousNextPatient EducationFor patient education information, see eMedicineHealth's patient education article Angina Pectoris.
PreviousProceed to Clinical Presentation  Contributor Information and DisclosuresAuthorXiushui (Mike) Ren, MD Cardiologist, The Permanente Medical Group; Associate Director of Research, Cardiovascular Diseases Fellowship, California Pacific Medical Center
Xiushui (Mike) Ren, MD is a member of the following medical societies: Alpha Omega Alpha, American College of Cardiology, and American Society of Echocardiography
Disclosure: Nothing to disclose.
Richard A Lange, MD Professor and Executive Vice Chairman, Department of Medicine, Director, Office of Educational Programs, University of Texas Health Science Center at San Antonio
Richard A Lange, MD is a member of the following medical societies: Alpha Omega Alpha, American College of Cardiology, American Heart Association, and Association of Subspecialty Professors
Disclosure: Nothing to disclose.
Jerry Balentine, DO Professor of Emergency Medicine, New York College of Osteopathic Medicine; Executive Vice President, Chief Medical Officer, Attending Physician in Department of Emergency Medicine, St Barnabas Hospital
Jerry Balentine, DO is a member of the following medical societies: American College of Emergency Physicians, American College of Osteopathic Emergency Physicians, American College of Physician Executives, American Osteopathic Association, and New York Academy of Medicine
Disclosure: Nothing to disclose.
Edward Bessman, MD, MBA Chairman and Clinical Director, Department of Emergency Medicine, John Hopkins Bayview Medical Center; Assistant Professor, Department of Emergency Medicine, Johns Hopkins University School of Medicine
Edward Bessman, MD, MBA is a member of the following medical societies: American Academy of Emergency Medicine, American College of Emergency Physicians, and Society for Academic Emergency Medicine
Disclosure: Nothing to disclose.
David FM Brown, MD Associate Professor, Division of Emergency Medicine, Harvard Medical School; Vice Chair, Department of Emergency Medicine, Massachusetts General Hospital
David FM Brown, MD is a member of the following medical societies: American College of Emergency Physicians and Society for Academic Emergency Medicine
Disclosure: Nothing to disclose.
Steven J Compton, MD, FACC, FACP, FHRS Director of Cardiac Electrophysiology, Alaska Heart Institute, Providence and Alaska Regional Hospitals
Steven J Compton, MD, FACC, FACP, FHRS is a member of the following medical societies: Alaska State Medical Association, American College of Cardiology, American College of Physicians, American Heart Association, American Medical Association, and Heart Rhythm Society
Disclosure: Nothing to disclose.
Daniel P Lombardi, DO Clinical Assistant Professor, New York College of Osteopathic Medicine; Attending Physician, Associate Department Director and Program Director, Department of Emergency Medicine, St Barnabas Hospital
Daniel P Lombardi, DO is a member of the following medical societies: American College of Emergency Physicians, American College of Osteopathic Emergency Physicians, and American Osteopathic Association
Disclosure: Nothing to disclose.
John A McPherson, MD, FACC, FAHA, FSCAI Associate Professor of Medicine, Division of Cardiovascular Medicine, Director of Cardiovascular Intensive Care Unit, Vanderbilt Heart and Vascular Institute
John A McPherson, MD, FACC, FAHA, FSCAI is a member of the following medical societies: Alpha Omega Alpha, American College of Cardiology, American Heart Association, Society for Cardiac Angiography and Interventions, Society of Critical Care Medicine, and Tennessee Medical Association
Disclosure: Abbott Vascular Corp. Consulting fee Consulting
Bekir H Melek, MD, FACC Assistant Professor of Clinical Medicine, Department of Medicine, Section of Cardiology, Tulane University School of Medicine
Disclosure: Nothing to disclose.
Gary Setnik, MD Chair, Department of Emergency Medicine, Mount Auburn Hospital; Assistant Professor, Division of Emergency Medicine, Harvard Medical School
Gary Setnik, MD is a member of the following medical societies: American College of Emergency Physicians, National Association of EMS Physicians, and Society for Academic Emergency Medicine
Disclosure: SironaHealth Salary Management position; South Middlesex EMS Consortium Salary Management position; ProceduresConsult.com Royalty Other
James V Talano, MD, MM, FACC Director of Cardiovascular Medicine, SWICFT Institute
Disclosure: Nothing to disclose.
Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference
Disclosure: Medscape Salary Employment
ReferencesWood S. Expanded FDA indication for Sapien valve. Medscape Medical News [serial online]. September 24, 2013;Accessed October 1, 2013. Available at http://www.medscape.com/viewarticle/811544.
FDA approval expands access to artificial heart valve for inoperable patients [press release]. Food and Drug Administration; September 23, 2013. [Full Text].
Tintinalli JE, Kelen GD, Stapczynski JS, eds. Valvular emergencies. In: 6th ed. Emergency Medicine: A Comprehensive Study Guide. New York: McGraw-Hill; 2004:54.
Bergler-Klein J. Natriuretic peptides in the management of aortic stenosis. Curr Cardiol Rep. Mar 2009;11(2):85-93. [Medline].
Townsend CM, et al. Sabiston Textbook of Surgery. 18th ed. Saunders; 2008:1841-1844..
Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, et al. Guidelines on the management of valvular heart disease (version 2012): The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. Oct 2012;33(19):2451-96. [Medline].
[Guideline] Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. Aug 1 2006;48(3):e1-148. [Medline].
Tzemos N, Therrien J, Yip J, Thanassoulis G, Tremblay S, Jamorski MT, et al. Outcomes in adults with bicuspid aortic valves. JAMA. Sep 17 2008;300(11):1317-25. [Medline].
Hughes BR, Chahoud G, Mehta JL. Aortic stenosis: is it simply a degenerative process or an active atherosclerotic process?. Clin Cardiol. Mar 2005;28(3):111-4. [Medline].
Roberts WC, Vowels TJ, Ko JM. Comparison of interpretations of valve structure between cardiac surgeon and cardiac pathologist among adults having isolated aortic valve replacement for aortic valve stenosis (+/- aortic regurgitation). Am J Cardiol. Apr 15 2009;103(8):1139-45. [Medline].
Kerstjens-Frederikse WS, Du Marchie Sarvaas GJ, et al. Left ventricular outflow tract obstruction: should cardiac screening be offered to first-degree relatives?. Heart. Aug 2011;97(15):1228-32. [Medline].
Lancellotti P, Magne J, Donal E, et al. Clinical outcome in asymptomatic severe aortic stenosis insights from the new proposed aortic stenosis grading classification. J Am Coll Cardiol. Jan 17 2012;59(3):235-43. [Medline].
Jander N, Minners J, Holme I, et al. Outcome of patients with low-gradient "severe" aortic stenosis and preserved ejection fraction. Circulation. Mar 1 2011;123(8):887-95. [Medline].
Rodrigues Tda R, Sternick EB, Moreira Mda C. Epilepsy or syncope? An analysis of 55 consecutive patients with loss of consciousness, convulsions, falls, and no EEG abnormalities. Pacing Clin Electrophysiol. Jul 2010;33(7):804-13. [Medline].
Topol EJ, Califf RM, et al, eds. Aortic valve disease. In: Textbook of Cardiovascular Medicine. Section Two. 3rd ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2007:Chap 23.
Zegdi R, Ciobotaru V, Huerre C, Allam B, Bouabdallaoui N, Berrebi A, et al. Detecting aortic valve bicuspidy in patients with severe aortic valve stenosis: high diagnostic accuracy of colour Doppler transoesophageal echocardiography. Interact Cardiovasc Thorac Surg. Jan 2013;16(1):16-20. [Medline]. [Full Text].
Zamorano JL, Badano LP, Bruce C, et al. EAE/ASE recommendations for the use of echocardiography in new transcatheter interventions for valvular heart disease. Eur Heart J. Sep 2011;32(17):2189-214. [Medline].
Piérard LA, Lancellotti P. Stress testing in valve disease. Heart. Jun 2007;93(6):766-72. [Medline]. [Full Text].
Messika-Zeitoun D, Aubry MC, Detaint D, Bielak LF, Peyser PA, Sheedy PF, et al. Evaluation and clinical implications of aortic valve calcification measured by electron-beam computed tomography. Circulation. Jul 20 2004;110(3):356-62. [Medline].
Shah RG, Novaro GM, Blandon RJ, Whiteman MS, Asher CR, Kirsch J. Aortic valve area: meta-analysis of diagnostic performance of multi-detector computed tomography for aortic valve area measurements as compared to transthoracic echocardiography. Int J Cardiovasc Imaging. Aug 2009;25(6):601-9. [Medline].
Bergler-Klein J, Klaar U, Heger M, Rosenhek R, Mundigler G, Gabriel H, et al. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation. May 18 2004;109(19):2302-8. [Medline].
Brown DW, Dipilato AE, Chong EC, Gauvreau K, McElhinney DB, Colan SD, et al. Sudden unexpected death after balloon valvuloplasty for congenital aortic stenosis. J Am Coll Cardiol. Nov 30 2010;56(23):1939-46. [Medline].
Agarwal A, Kini AS, Attanti S, Lee PC, Ashtiani R, Steinheimer AM, et al. Results of repeat balloon valvuloplasty for treatment of aortic stenosis in patients aged 59 to 104 years. Am J Cardiol. Jan 1 2005;95(1):43-7. [Medline].
Rahimtoola SH. Choice of prosthetic heart valve in adults an update. J Am Coll Cardiol. Jun 1 2010;55(22):2413-26. [Medline].
[Best Evidence] Stassano P, Di Tommaso L, Monaco M, Iorio F, Pepino P, Spampinato N, et al. Aortic valve replacement: a prospective randomized evaluation of mechanical versus biological valves in patients ages 55 to 70 years. J Am Coll Cardiol. Nov 10 2009;54(20):1862-8. [Medline].
Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. Oct 21 2010;363(17):1597-607. [Medline].
Clavel, MA, et al. Comparison Between Transcatheter and Surgical Prosthetic Valve Implantation in Patients With Severe Aortic Stenosis and Reduced Left Ventricular Ejection Fraction. Circulation. Nov 9 2010Vol;. 122 No.19.
Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. Jun 9 2011;364(23):2187-98. [Medline].
Daneault B, Kirtane AJ, Kodali SK, et al. Stroke associated with surgical and transcatheter treatment of aortic stenosis: a comprehensive review. J Am Coll Cardiol. Nov 15 2011;58(21):2143-50. [Medline].
Herrmann HC, Pibarot P, Hueter I, Gertz ZM, Stewart WJ, Kapadia S, et al. Predictors of Mortality and Outcomes of Therapy in Low-Flow Severe Aortic Stenosis: A Placement of Aortic Transcatheter Valves (PARTNER) Trial Analysis. Circulation. Jun 11 2013;127(23):2316-26. [Medline].
Hahn RT, Pibarot P, Stewart WJ, et al. Comparison of transcatheter and surgical aortic valve replacement in severe aortic stenosis: a longitudinal study of echocardiography parameters in cohort A of the PARTNER Trial (Placement of Aortic Transcatheter Valves). J Am Coll Cardiol. Jun 25 2013;61(25):2514-21. [Medline].
[Guideline] Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. May 21 2003;289(19):2560-72. [Medline].
[Guideline] Nishimura RA, Carabello BA, Faxon DP, et al. ACC/AHA 2008 Guideline Update on Valvular Heart Disease: Focused Update on Infective Endocarditis: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. August 2008;52:676-85. [Medline].
Moura LM, Ramos SF, Zamorano JL, Barros IM, Azevedo LF, Rocha-Gonçalves F, et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol. Feb 6 2007;49(5):554-61. [Medline].
Chan KL, Teo K, Dumesnil JG, Ni A, Tam J. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. Jan 19 2010;121(2):306-14. [Medline].
Rossebo AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. Sep 25 2008;359(13):1343-56. [Medline].
Cowell SJ, Newby DE, Prescott RJ, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. Jun 9 2005;352(23):2389-97. [Medline].
[Guideline] Wann LS, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS Focused Update on the Management of Patients With Atrial Fibrillation (Update on Dabigatran): A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. Mar 15 2011;123(10):1144-50. [Medline]. [Full Text].
[Best Evidence] Bagur R, Webb JG, Nietlispach F, Dumont E, De Larochellière R, Doyle D, et al. Acute kidney injury following transcatheter aortic valve implantation: predictive factors, prognostic value, and comparison with surgical aortic valve replacement. Eur Heart J. Apr 2010;31(7):865-74. [Medline]. [Full Text].
Eltchaninoff H, Prat A, Gilard M et al,. Transcatheter aortic valve implantation: early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur Heart Jl. 2011;32:191–197.
Lefevre T, Kappetein AP, Wolner E, et al. One year follow-up of the multi-centre European PARTNER transcatheter heart valve study. Eur Heart Jl. 2011;32:148–157.
Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. Oct 21 2010;363(17):1597-607. [Medline].
Prasad Y, Bhalodkar NC. Aortic sclerosis--a marker of coronary atherosclerosis. Clin Cardiol. Dec 2004;27(12):671-3. [Medline].
[Best Evidence] Rosenhek R, Zilberszac R, Schemper M, Czerny M, Mundigler G, Graf S, et al. Natural history of very severe aortic stenosis. Circulation. Jan 5 2010;121(1):151-6. [Medline].
Tamburino C, Capodanno D, Ramondo A, Petronio AS, Ettori F, Santoro G, et al. Incidence and predictors of early and late mortality after transcatheter aortic valve implantation in 663 patients with severe aortic stenosis. Circulation. Jan 25 2011;123(3):299-308. [Medline].
Zahn R, Gerckens U, EGrube E et al. Transcatheter aortic valve implantation: first results from a multi-centre real-world registry. Eur Heart Jl. 2011;32:198–204.
Zajarias A, Cribier AG. Outcomes and safety of percutaneous aortic valve replacement. J Am Coll Cardiol. May 19 2009;53(20):1829-36. [Medline].
Â
 Calcific aortic stenosis (parasternal long-axis and short-axis views). Stenotic aortic valve (macroscopic appearance). Table 1. Common Causes of Aortic Stenosis Among Patients Requiring SurgeryTable 2. ACC/AHA Recommendations for Echocardiography (Imaging, Spectral, and Color Doppler) in Aortic StenosisTable 3. Criteria for Determining Severity of Aortic StenosisTable 4. Recommendations for Cardiac Catheterization in Aortic StenosisTable 5. Recommendations for Aortic Valve Replacement in Aortic StenosisTable 1. Common Causes of Aortic Stenosis Among Patients Requiring SurgeryAge Age >70 years (n=322) Bicuspid AV (50%)
Calcific aortic stenosis (parasternal long-axis and short-axis views). Stenotic aortic valve (macroscopic appearance). Table 1. Common Causes of Aortic Stenosis Among Patients Requiring SurgeryTable 2. ACC/AHA Recommendations for Echocardiography (Imaging, Spectral, and Color Doppler) in Aortic StenosisTable 3. Criteria for Determining Severity of Aortic StenosisTable 4. Recommendations for Cardiac Catheterization in Aortic StenosisTable 5. Recommendations for Aortic Valve Replacement in Aortic StenosisTable 1. Common Causes of Aortic Stenosis Among Patients Requiring SurgeryAge Age >70 years (n=322) Bicuspid AV (50%)Postinflammatory (25%)
Degenerative (18%)
Unicommissural (3%)
Hypoplastic (2%)
Indeterminate (2%)
Degenerative (48%)
Bicuspid (27%)
Postinflammatory (23%)
Hypoplastic (2%)
Table 2. ACC/AHA Recommendations for Echocardiography (Imaging, Spectral, and Color Doppler) in Aortic StenosisIndication Class Diagnosis and assessment of severity of aortic stenosisIAssessment of LV size, function, and/or hemodynamicsIReevaluation of patients with known aortic stenosis with changing symptoms or signsIAssessment of changes in hemodynamic severity and ventricular function in patients with known aortic stenosis during pregnancyIReevaluation of asymptomatic patients with severe aortic stenosisIReevaluation of asymptomatic patients with mild to moderate aortic stenosis and evidence of LV dysfunction or hypertrophyIIaRoutine reevaluation of asymptomatic adult patients with mild aortic stenosis who have stable physical signs and normal LV size and function IIITable 3. Criteria for Determining Severity of Aortic StenosisSeverity Mean gradient (mm Hg) Aortic valve area (cm2) Mild>1.5Moderate25-401-1.5Severe>40
(or 2/m2 body surface area)
Critical>80Table 4. Recommendations for Cardiac Catheterization in Aortic StenosisIndication Class Coronary angiography before aortic valve replacement in patients at risk for coronary artery diseaseIAssessment of severity of aortic stenosis in symptomatic patients when aortic valve replacement is planned or when noninvasive tests are inconclusive or a discrepancy exists in the clinical findings regarding the severity of aortic stenosis or the need for surgery ICoronary angiography before aortic valve replacement in patients for whom a pulmonary autograft (Ross procedure) is contemplated and the origin of the coronary arteries was not identified by noninvasive tests IWith infusion of dobutamine, can be useful for evaluation of patients with low-flow/low-gradient aortic stenosis and LV dysfunctionIIaNot recommended for hemodynamic measurements for assessment of aortic stenosis severity when noninvasive techniques are adequate and concord with clinical findings IIINot recommended for hemodynamic measurements for assessment of LV function and aortic stenosis severity in asymptomatic patientsIIITable 5. Recommendations for Aortic Valve Replacement in Aortic StenosisIndication Class Symptomatic patients with severe aortic stenosisIPatients with severe aortic stenosis undergoing coronary artery bypass surgeryIPatients with severe aortic stenosis undergoing surgery on the aorta or other heart valvesIPatients with severe aortic stenosis and LV systolic dysfunction (ejection fraction IPatients with moderate aortic stenosis undergoing coronary artery bypass surgery or surgery on the aorta or other heart valvesIIaPatients with mild aortic stenosis undergoing coronary artery bypass surgery when there is evidence that progression may be rapid, such as moderate-to-severe valve calcificationIIbAsymptomatic patients with severe aortic stenosis and abnormal response to exercise (eg, hypotension)IIbAsymptomatic patients with severe aortic stenosis and a high likelihood of rapid progression (based on age, calcification, and coronary artery disease) or if surgery might be delayed at the time of symptom onsetIIbAsymptomatic patients with extremely severe aortic stenosis (valve area less than 0.6 cm2, mean gradient greater than 60 mm Hg, and jet velocity greater than 5 m per second) if the patient’s expected operative mortality is 1% or lessIIbAVR is not useful for prevention of sudden death in asymptomatic patients with none of the findings listed under asymptomatic patients with severe aortic stenosisIII

  View Table List Read more about Aortic Stenosis on MedscapeRelated Reference Topics
 View Table List Read more about Aortic Stenosis on MedscapeRelated Reference TopicsAortic Stenosis Pathology
Pediatric Supravalvar Aortic Stenosis
Pediatric Valvar Aortic Stenosis
Related News and Articles
Disease Prevalence and Number of Candidates for Transcatheter Aortic Valve Replacement
TAVI No Better Than Standard Surgery for Severe Aortic Stenosis: Study
Overview of the 2012 Food and Drug Administration Circulatory System Devices Panel of the Medical Devices Advisory Committee
Meeting on the Edwards SAPIEN Transcatheter Heart Valve for High-Risk Aortic Stenosis Patients
Medscape Reference © 2011 WebMD, LLC, Aortic Stenosis






0 comments:
Post a Comment