In 1994, Dake et al first reported the use of thoracic "stent-grafts" for the treatment of descending thoracic aortic aneurysms in patients who were believed to be at excessive risk for conventional open surgery.[1] These authors showed that thoracic stent grafts, otherwise known as Thoracic EndoVascular Aortic Repair (TEVAR), could be performed from a technical standpoint with relatively low morbidity; however, they noted that long-term follow-up would be required. The initial stent grafts were actually constructed by the implanting physicians themselves and were later restricted to devices under investigational study.
In 2005, The US Food and Drug Administration (FDA) approved the first commercially available thoracic stent graft, the W. L. Gore TAG endograft system (Flagstaff, Ariz), and, in 2008, the US FDA approved the Cook Zenith TX2 (Bloomington, Ind)[2] and the Medtronic Talent (Santa Rosa, Calif) thoracic endograft systems; all 3 were approved for use in the treatment of thoracic aortic aneurysms.
The randomized clinical trial that led to the approval of the TAG device demonstrated that, in patients with appropriate anatomy, TEVAR could be performed with lower operative mortality versus open surgery (2.1% vs 11.7%) and with less spinal cord ischemia (3% vs 14%), respiratory insufficiency (4% vs 20%), and renal failure (1% vs 13 %).[3] However, more vascular access–related complications occurred in the TEVAR group. Importantly, a small percentage of patients who were undergoing TEVAR did not have their aneurysms entirely excluded from the aortic circulation at 1- and 2-year follow-up. Once more, these authors maintained that all patients undergoing TEVAR required close long-term follow-up.
Dake et al and investigators in Europe later showed TEVAR could be performed from a technical standpoint in patients with descending thoracic aortic dissections (patients with tears in the wall of their aortas).[4] Nonetheless, ongoing studies are still trying to identify which patients with thoracic aortic dissection can benefit from TEVAR. Patients with complicated dissections, including cases of malperfusion (where a blood supply is impeded by flaps of aortic tissue caused by the dissection), appear to benefit from TEVAR to seal the site of dissection and reappose aortic wall layers.[4]
Note that historically open surgical approaches in patients with malperfusion have had a high mortality rate.[5] Accordingly, in the United States, a clinical trial is examining TEVAR for patients with complicated dissection (STABLE trial, Cook Inc, Bloomington, Ind). It would seem logical that TEVAR could also be used in patients with uncomplicated dissections. To address this question, a prospective randomized clinical trial has recently been completed in Europe, the INSTEAD trial (INvestigation of STEnt grafts in patients with type B aortic dissection), though the results have not yet been published.[6]
Since the 2005 FDA approval of the TAG device for thoracic aneurysms, the use of endovascular stent grafts for thoracic aortic disease has increased dramatically. In recent years, surgeons have devised novel techniques to facilitate the use of TEVAR in higher-risk patients. Branch vessels that would have been occluded by the stent grafts can often be bypassed, and the diameter of larger areas of aorta can often be reduced to accommodate stent grafts.[5, 7, 8] Indeed in the authors' own experience at the University of Florida, a several-fold increase has been seen in TEVAR over open cases in the past few years, as shown in the graph below.
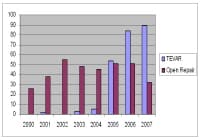 University of Florida/Shands Hospital in Gainesville, Florida, experience with open repair and Thoracic EndoVascular Aortic Repair (TEVAR) repair of thoracic aortic disease with a shift toward TEVAR. NextIndications
University of Florida/Shands Hospital in Gainesville, Florida, experience with open repair and Thoracic EndoVascular Aortic Repair (TEVAR) repair of thoracic aortic disease with a shift toward TEVAR. NextIndicationsDescending thoracic aortic aneurysm
The official FDA-approved "on-label" indication for the commercially available stent grafts currently available in the United States (W. L. Gore TAG, Cook TX2, Medtronic Talent Thoracic) are for patients with descending thoracic aortic aneurysms that are at least 2 times greater than the adjacent aorta. Furthermore, sufficient aorta of normal dimensions must be present on either side of the aneurysm (so-called landing zones) for the stent graft to adhere to the aortic walls. Of note, several patients were specifically excluded from the randomized trial that led to the TAG approval, including patients with acute or chronic dissections, patients with ruptured or infected (mycotic) aneurysm, and patients with connective tissue disorders such as Marfan disease.[3] Nonetheless, at the discretion of individual surgeons, TEVAR has been performed "off label" in other patient groups, especially at times of emergency. As an example, in cases of aortic rupture secondary to massive blunt traumatic injury (eg, motor vehicle accidents), TEVAR has been used as a less-invasive method to perform aortic repair in patients with multiple, extensive injuries.[9]Focal penetrating ulcer: Patients with focal penetrating ulcers in their thoracic aorta are another group in which TEVAR may prove beneficial because these patients have defined, limited areas of their thoracic aorta where a loss in the integrity of the endothelium leads to potentially life-threatening rupture. Coverage of the ulcer with a stent graft can be performed with minimal morbidity. However, patients with penetrating ulcers often have extensive peripheral vascular disease, which may limit the use of TEVAR in these patients.[6, 10]
Complicated descending thoracic aortic dissection: Some evidence indicates that TEVAR may be the optimal treatment of patients with complicated descending thoracic aortic dissections compared with open surgery.[4, 5] In the United States, the Zenith TX-D endograft system (Cook, Bloomington, Ind) is currently under investigation for complicated dissection.
PreviousNextContraindicationsPatients undergoing TEVAR must have suitable anatomy in order to deploy the endografts. Specifically, patients who have undergone TEVAR must have appropriate landing zones, typically 2 cm of suitable aortic diameter that can accommodate an endograft on either side of the thoracic aortic pathology.
Patients who will have branch vessels occluded by the stent graft (including the celiac and subclavian or carotid arteries) may not be candidates; however, these branch vessels can often be bypassed to create landing zones in so-called hybrid techniques.[11]
Patients with connective tissue disorders in whom a high likelihood exists of further tissue degeneration (eg, Marfan disease) were specifically excluded from the trials that led to the FDA approval of the devices available in the United States (TAG, TX2, Talent).
PreviousNextAnesthesiaBoth general anesthesia and continuous spinal anesthesia have been used by these authors. Large-bore intravenous access for volume infusion is mandatory, along with continuous arterial pressure monitoring.
More recently, the authors have used spinal drains routinely in the patients undergoing Thoracic EndoVascular Aortic Repair (TEVAR); they are placed to drain at 10 mm Hg (15 cm H2 O) pressure for 24 hours and are then removed at 48 hours. Other surgical groups reserve spinal drains for patients deemed to be at highest risk for spinal ischemia (patients undergoing extensive coverage of the thoracic aorta and patients with a history of prior abdominal aortic aneurysm repair). Patients require vigilant monitoring in the early postoperative period for the development of neurologic deficits. Delayed neurologic deficits can still be reversed with elevation of systemic arterial pressure and drainage of spinal fluid.[12]
PreviousNextEquipmentThe operative suite should have a fixed imaging system, as shown below, to ensure optimal results from TEVAR.
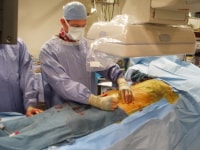 Cannulation of left common femoral artery with a 4F catheter in an endovascular suite with fixed overhead imaging system.
Cannulation of left common femoral artery with a 4F catheter in an endovascular suite with fixed overhead imaging system. Hybrid operating rooms provide optimal imaging technology for endovascular procedures and also are sufficiently large to accommodate open surgery for cases in which it is required. Ceiling mounted monitors showing the patient’s vital signs and preoperative CT scans are recommended.
The operating table should allow free access to the imaging C-arm below and be long enough to accommodate the long guidewires that are used via the femoral artery access points.
PreviousNextPositioningThe patient remains in the supine position with the arms in "surrender" position to allow lateral angiography.
When brachial artery access is required, the arms are placed at 90-degree angles and prepared and draped in the operating field.
Bilateral femoral artery access points are prepared along with the abdomen to the level of the nipples.
The entire field is covered with iodine-impregnated adhesive wrap as shown below.
 Cannulation of left common femoral artery with a 4F catheter in an endovascular suite with fixed overhead imaging system. PreviousNextTechnique
Cannulation of left common femoral artery with a 4F catheter in an endovascular suite with fixed overhead imaging system. PreviousNextTechniqueThe key to a successful procedure begins with meticulous preoperative planning to determine the precise size of the endograft, its length, and its relation to critical branch vessels, as in the image below. Access sites are chosen based on CT scan reconstruction of the anatomy. The femoral artery must be of sufficient diameter to pass the endograft; otherwise, a conduit (10-mm Dacron tube graft) needs to be attached to the larger iliac artery via a retroperitoneal incision.[13]
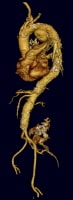 Three-dimensional CT reconstruction of thoracic aorta with aneurysm in the arch aorta.
Three-dimensional CT reconstruction of thoracic aorta with aneurysm in the arch aorta. Close cooperation with the anesthesia team is required. Place spinal drainage catheters prospectively in patients, especially in those at highest risk for spinal injury (patients undergoing complete thoracic aortic coverage or with a previous abdominal aneurysm repair).
Position and sterilely prepare the patient as outlined above (see Positioning).
Obtain percutaneous femoral arterial access on the side that will not be used for delivering the endograft, as shown in the image below. Insert a 5F introducer sheath, and pass a pigtail catheter over a 0.035-in guidewire. Confirm catheter position with a brief injection of contrast.
 Cannulation of left common femoral artery with a 4F catheter in an endovascular suite with fixed overhead imaging system.
Cannulation of left common femoral artery with a 4F catheter in an endovascular suite with fixed overhead imaging system. 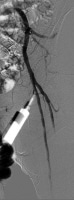 Confirmation angiogram showing appropriate cannulation of the left common femoral artery.
Confirmation angiogram showing appropriate cannulation of the left common femoral artery. After meticulous removal of all air bubbles to avoid air emboli, connect the pigtailed catheter to a mechanical injection system like the one shown below.
 Automated contrast injection system attached to the 5F pigtail catheter inserted via the left common femoral artery.
Automated contrast injection system attached to the 5F pigtail catheter inserted via the left common femoral artery. Perform an arch arteriogram, typically at 30 degrees left anterior obliquity to delineate the arch vessels, as depicted in the image below.
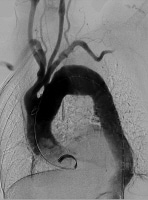 Baseline aortogram showing arch anatomy.
Baseline aortogram showing arch anatomy. Obtain open exposure of the contralateral common femoral artery that will deliver the stent graft, as shown below, and pass a vessel loop for proximal control. Of note, if the common femoral artery is too small to pass the large delivery catheters, then sew a Dacron graft "conduit" to the side of the iliac artery via a retroperitoneal incision and use it to pass the stent graft into position.
 Retroperitoneal incision to access the right common iliac artery.
Retroperitoneal incision to access the right common iliac artery. 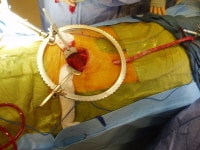 Dacron conduit attached to the right common iliac artery brought out below through a separate incision.
Dacron conduit attached to the right common iliac artery brought out below through a separate incision. Directly cannulate this conduit using an 18-ga needle, and pass a 0.035-in guidewire into the ascending aorta under fluoroscopic guidance. Then exchange this flexible wire for a "superstiff" wire (eg, Lunderquist) that will be used to provide a rail to deliver the stent graft into position.
On the back table, prior to use, meticulously prepare the endograft delivery system (shown below) according to manufacturer instructions to remove air bubbles and to ensure proper delivery of the endograft.
 Thoracic endograft being prepared on the back table prior to deployment.
Thoracic endograft being prepared on the back table prior to deployment. Use the radiographic markers on the endograft to position it exactly where it is to be deployed, and obtain confirmation angiographic images as necessary, as shown below. Sufficient landing zone (2 cm) is required to seat the endograft; occasionally, this requires deploying the endograft across the left subclavian artery. Deploy the thoracic endograft.
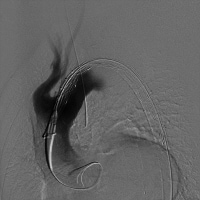 Predeployment angiogram showing the endograft in the arch aorta.
Predeployment angiogram showing the endograft in the arch aorta. Perform balloon dilation of the endograft as appropriate to ensure apposition of the endograft to the wall of the thoracic aorta in order to minimize the potential for endoleaks around the endograft.
Obtain completion angiographic images, as depicted in the images below. If necessary, the left subclavian artery can be bypassed and/or occluded with coil embolization to prevent endoleaks.
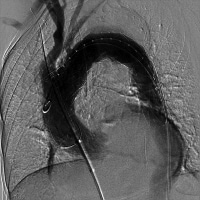 Angiogram performed following endograft deployment.
Angiogram performed following endograft deployment. 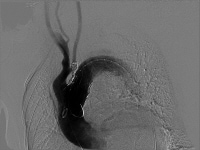 Coil embolization of left subclavian artery to prevent endoleak.
Coil embolization of left subclavian artery to prevent endoleak. Carefully remove delivery catheters and sheaths with the contralateral guidewire remaining in position. The "delivery" femoral or iliac artery is routinely repaired.
Remove the guidewire from the "imaging femoral artery" and seal the femoral artery access point.
Confirm distal pulses prior to leaving the operating suite.
Transfer patients to the cardiac intensive care unit for monitoring of neurologic and hemodynamic function.
PreviousNextPearlsMultidisciplinary collaboration between skilled endovascular therapists and cardiovascular surgeons is critical to safe and successful development of an endovascular aortic treatment program. Although the incidence of intraoperative surgical conversion remains low, late complications and remedial secondary procedures may require complex thoracic aortic reconstructions. Despite the seeming simplicity of these procedures, unforeseen anatomic and device-related complexities can transform these cases into some of the most complicated endovascular procedures that require advanced endovascular skills in order to bring the procedure to a safe and successful conclusion.
Maintaining proper guidewire access throughout the procedure is critical to the safety of the procedure. The guidewire is analogous to "proximal" control of a blood vessel during open vascular surgery. As long as guidewires remain in position, life-saving occlusion balloons can be passed proximal to iliac or femoral artery sites that have been known to tear during sheath retrieval.
Catheter and guidewire "hygiene" is important to avoid air embolism and thromboembolism of clot and fibrin debris that tend to collect around the guidewires. The guidewires should be wiped, and the catheters should be flushed frequently with heparinized saline during the procedure.
Implanting appropriate-sized endografts is critical; if too large a device is deployed, it can collapse or infold and cause aortic occlusion.
PreviousNextComplicationsAs with any surgical therapy, complications are associated with TEVAR. The most severe complications in the W.L. Gore TAG pivotal clinical trial included stroke (4%), paraplegia/paraparesis (3%), peripheral vascular injury (14%), and death (2%).[3] Stroke can occur because the guidewires that are placed in the aortic arch to direct the endografts into position can dislodge a thrombus or atheromata, which can embolize via the cerebral vasculature to the brain. For that matter, emboli can also lead to limb and mesenteric ischemia. Spinal ischemia occurs when the intercostal blood vessels supplying the spinal cord are covered by the stent grafts. Paraplegia occasionally can be reversed by elevating the blood pressure and draining spinal fluid.[12]
Because of the large size of the stent grafts, they can damage the femoral and iliac arteries as they are passed into position. The most worrisome concern is complete avulsion of the arteries, which can be controlled with balloon occlusion; this is the reason guidewires must be left in position until the very end of the case. Concern for these rare but major vascular catastrophes is one reason TEVAR should be performed only by surgeons experienced with open repair techniques.
Endoleaks occur when the aneurysm is not completely isolated from the bloodstream; this was found in 6% and 9% of patients at 1 and 2 years, respectively, in the TAG trial.[3] Accordingly, emphasizing that patients undergoing TEVAR require lifelong follow-up with annual CT scans is important. The classification system for endoleaks is as follows:
Type I endoleaks occur when the seal on either end of the stent graft is incomplete.Type II endoleaks occur because of back bleeding from smaller vessels (typically intercostals) that are covered with the endograft.Type III endoleaks occur when leak occurs between overlapping stent grafts.Type IV endoleaks were seen with earlier stent grafts when porosity of the graft material led to seepage of blood components through the graft walls, resulting in tension within the excluded aneurysmal segment (rarely seen today). Previous, Thoracic Endovascular Aortic Repair





0 comments:
Post a Comment